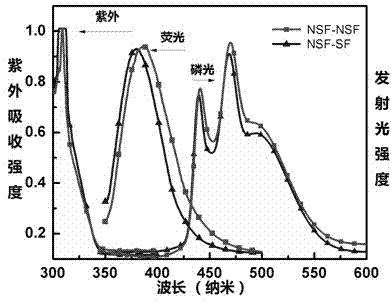
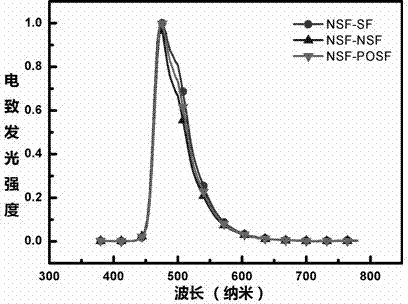
9,9'- connected host material based on 4,4'-difluorene structure and application thereof
A technology of host materials and doping materials, which is applied in the field of host materials connected at 9,9'-position, can solve the problems of decreased energy transmission efficiency, low triplet state, and high device startup voltage, and achieves high-efficiency electroluminescence performance, high The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 110
[0024] Example 11 Synthesis of 110-phenyl-10 hydrogen-spiro[acridine-9,9'-fluorene]-4-ylboronic acid pinacol borate (abbreviated as intermediate 1)
[0025] Synthesis of intermediate (A)
[0026]
[0027] Under the protection of Ar gas, add o-bromotriphenylamine (1.6g; 5mmol) and 60ml of anhydrous and oxygen-free tetrahydrofuran into a 200ml Chirank bottle, cool to -78°C at low temperature, and add 3ml of n-butyllithium (2M; 6mmol) dropwise. ), after reacting at low temperature for 1 hour, dropwise added 4-bromo-9-fluorenone (1.2 g; 5 mmol) dissolved in anhydrous and oxygen-free tetrahydrofuran under the protection of Ar gas, and continued the reaction at low temperature for 1 hour, then took it out and stirred overnight at room temperature. The reaction was then quenched with saturated ammonium chloride solution and dried over anhydrous sodium sulfate. After the solvent was evaporated by a rotary evaporator, the remaining solid was dissolved in acetic acid, refluxed at 12...
Embodiment 24
[0030] Example 2 Synthesis of 4'-bromo-5-phenylspiro[phosphine-10,9'fluorene] 5-oxide (abbreviated as intermediate product B) Synthesis of o-bromotriphenylphosphine oxygen
[0031]
[0032]Under the protection of Ar gas, o-dibromobenzene (1.2 g; 5 mmol) and 40 ml of dehydrated and deoxygenated tetrahydrofuran were added to a 200 ml Chirac flask, and cooled to 0° C. in an ice bath. Add 4ml of Grignard reagent (1.8M; 7.2mmol) dropwise and react at low temperature for 1h, then add diphenylphosphorus chloride (0.7ml; 6mmol) dropwise, keep it at 0°C for 1h, then take it out to react overnight at room temperature. Saturated ammonium chloride solution was quenched, and after the solvent was evaporated by a rotary evaporator, the product was separated and purified by column chromatography to obtain 0.8 g (58%) of the product o-bromotriphenylphosphine oxide.
[0033]
[0034] Using o-bromotriphenylphosphine oxide instead of o-bromotriphenylamine, using the same synthesis conditio...
Embodiment 3
[0035] Synthesis of Example 34-(4-pinacol borate ester-9 hydrogen-fluoren-9-yl)-nitrogen, nitrogen-diphenylaniline (intermediate 2 for short)
[0036]
[0037] Synthesis of Intermediate C
[0038]
[0039] Under the protection of nitrogen, put 4-bromo-triphenylamine (1.6g; 5mmol) into a 200ml Chirank bottle, then add 40ml of ether, put it into a low-temperature reactor and cool it to -78°C, and add n-butyllithium dropwise 3ml (2M; 6mmol), after low temperature reaction for 1h, slowly drop 4-bromo-9-fluorenone (1.3g; 5mmol) which has been dissolved in anhydrous oxygen-free ether into the Schrank bottle, and react for 1h take out. Slowly return to room temperature, pass dry ammonia gas (200ml) into the reaction bottle, remove the gas device, add 200mg of lithium wire, react for 0.5h, open the bottle mouth to volatilize the ammonia gas. The remaining solvent was quenched with saturated ammonium chloride, extracted with ethyl acetate, evaporated to dryness with a rotary ev...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum power efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com