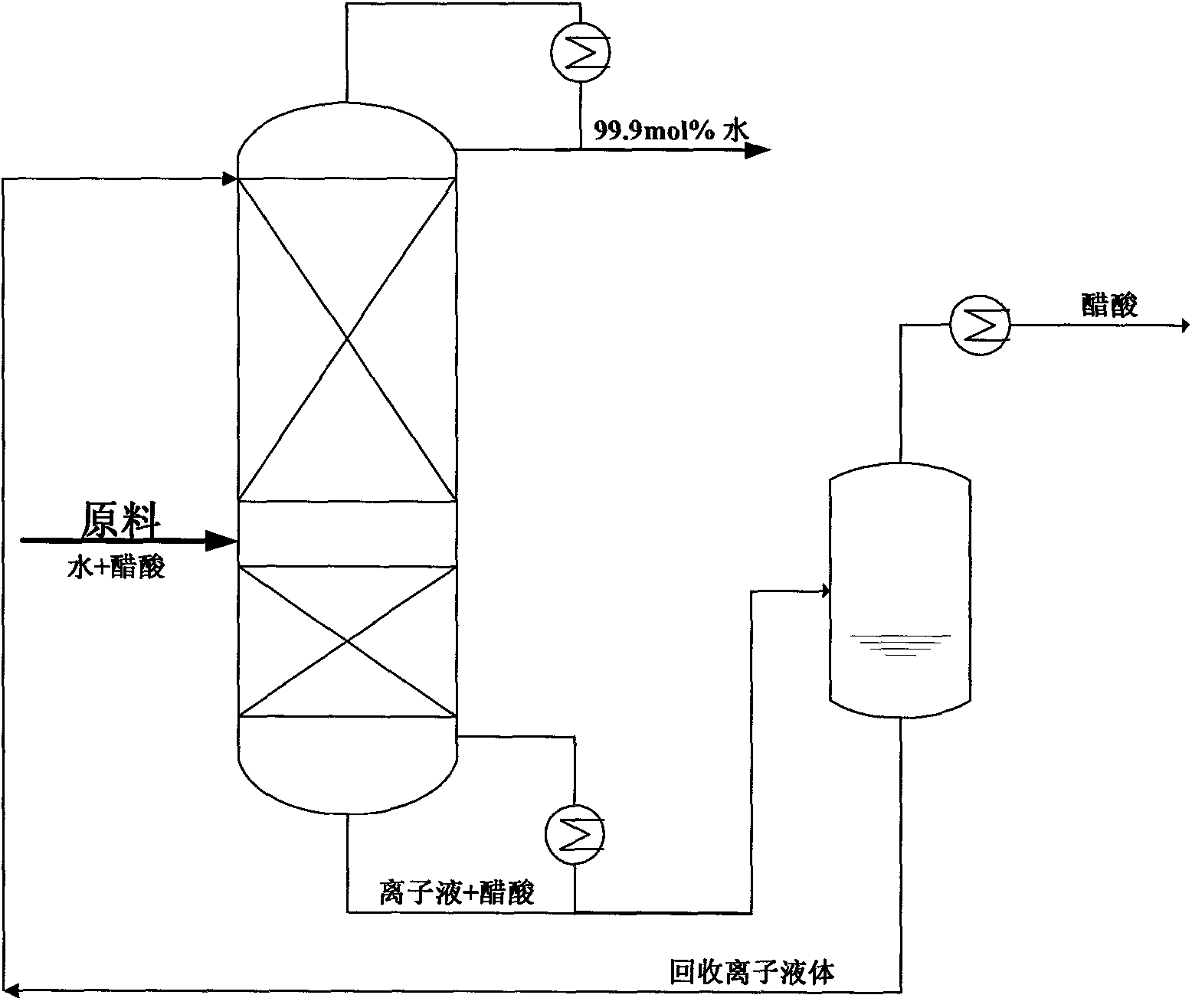
Ionic liquid extractive distillation method for separating acetic acid and water
The technology of ionic liquid and separation method is applied in the field of extractive distillation method to separate acetic acid aqueous solution, which can solve the problems of secondary environmental pollution, large solvent loss, disproportionation, etc., and achieves the effect of high safety index and simplified process and operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
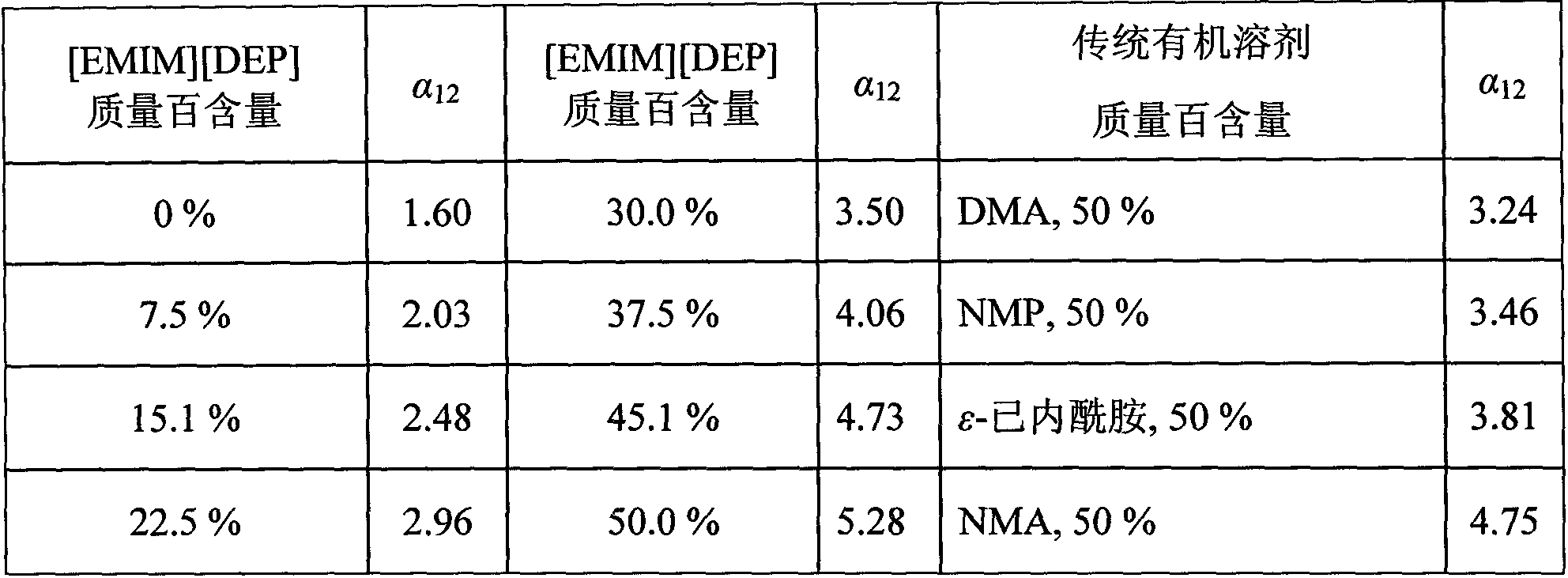
[0017] The improved Rose vapor-liquid balance kettle is used to measure the equal-pressure vapor-liquid balance data, the pressure is controlled by a two-stage pressure precision controller, the pressure fluctuation range is (±0.03) kPa, and the balance temperature is measured by a calibrated precision mercury thermometer (accuracy 0.1K). . At 101.32kPa, 1-ethyl-3-methylimidazolium diethyl phosphate ([EMIM][DEP]) was added to aqueous acetic acid (x 水 =0.9), its addition is difficult to separate point (x 水 =0.9) relative volatility α 12 The impact, see Table 1:
[0018] Table 1
[0019]
[0020] It can be seen from Table 1 that the ionic liquid [EMIM][DEP] has a significant salting-out effect, has a high affinity to the acetic acid component, and has a relative volatility from 1.60 (no ionic liquid) to 5.28 (about 50% of the ionic liquid mass content), which is no 3.3 times that of the ionic liquid system, relative volatility α 12 Much higher than the ionic liquid-free ...
Embodiment 2
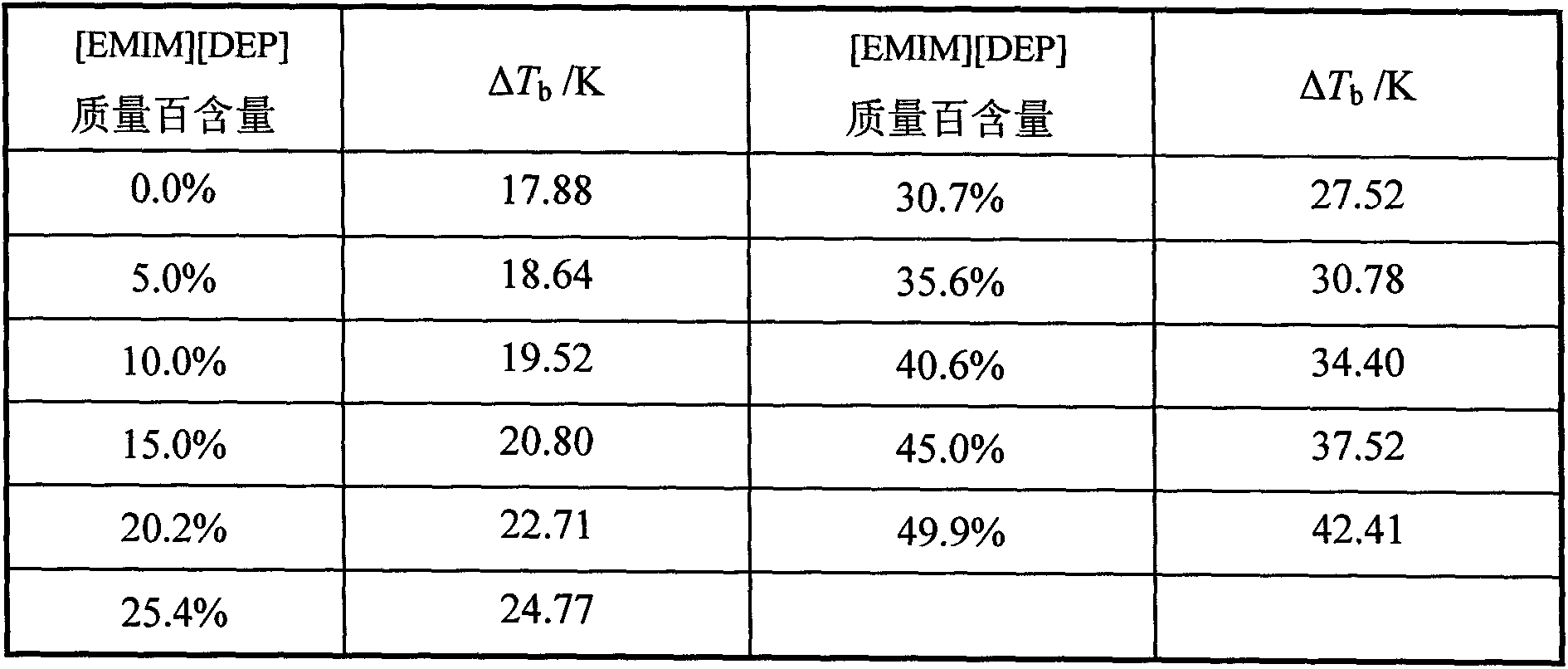
[0022] Adopt the measurement data of the Rose vapor-liquid balance kettle in the embodiment 1, control the pressure with the secondary pressure precision controller, under 101.32kPa, add the ionic liquid [EMIM][DEP] in the acetic acid aqueous solution, investigate its addition to the component Boiling point difference ΔT b The impact, see Table 2:
[0023] Table 2
[0024]
[0025] It can be seen from Table 2 that with the increase of the content of ionic liquid [EMIM][DEP], the boiling point difference of water+acetic acid system increases from 17.88K (without ionic liquid) to 42.41K (about 50% of the mass content of ionic liquid), indicating that the acetic acid aqueous solution Distillation separation becomes easier.
Embodiment 3
[0027] Adopt the Rose vapor-liquid equilibrium kettle measurement data among the embodiment 1, under 101.32kPa, 1-butyl-3-methylimidazole dibutyl phosphate ([BMIM][DBP]) is added aqueous acetic acid (x 水 =0.9), its addition is difficult to separate point (x 水 =0.9) relative volatility, see Table 3:
[0028] table 3
[0029]
[0030] It can be seen from Table 3 that there is an obvious salting-out effect in ionic liquids, and the relative volatility α 12 Much higher than the ionic liquid-free system, when the mass content of ionic liquid reaches 57.08%, the relative volatility α 12 As high as 9.81, 6.1 times that of the ionic liquid-free system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com