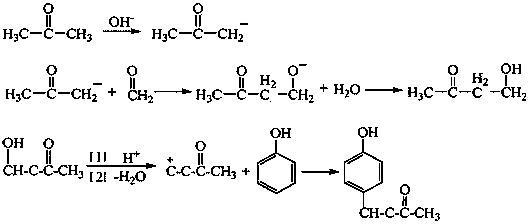
Synthesis process of raspberry ketone
A technology for raspberry ketone and synthesis process, which is applied in the field of raspberry ketone synthesis technology, can solve the problems of large consumption of raw material acetone, complicated post-processing, incomplete reaction, etc., and achieves saving resin consumption, reducing solvent recovery burden, and reducing production. dangerous effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
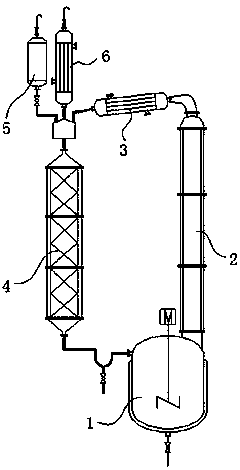
[0032] The synthesis of embodiment 1 butanone alcohol
[0033] The synthesis of butanol alcohol, the main equipment of technique is as figure 1 As shown, the raw material acetone and part of citric acid are put into the reactor 1, the reactor 1 starts heating and stirring, and the θ-ring packed fixed-bed reactor 4 starts heating. At a certain temperature, the acetone vapor rises from the return side pipe 2 and enters the packing column after being condensed by the condenser 3, and the acetone is continuously evaporated and refluxed. After the temperature stabilizes, the formaldehyde lye metering tank 5 starts to add dropwise, and the reaction is carried out in the packed column. The mixed solution is continuously returned to the reactor 1, the redundant alkali in the mixed solution is neutralized by citric acid in the still, the product butanone alcohol remains in the still because of its high boiling point, and the acetone continues to evaporate and reflux. When the formald...
Embodiment 2
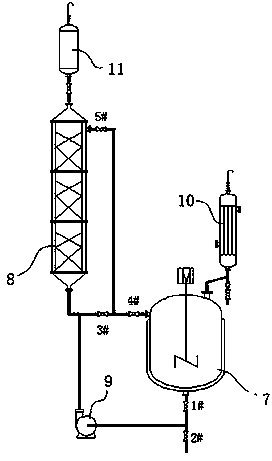
[0039] The synthesis of embodiment 2 raspberry ketone
[0040] The synthetic technique of raspberry ketone is as figure 2 As shown, the dissolved phenol and toluene are dropped into the reactor 7, the reactor 7 and the resin layer fluidized bed reactor 8 start heating, open the 1#, 5#, 4# valves, the centrifugal pump 9, and close the 2#, 3 #valve. The reaction solution flows through the resin layer from bottom to top, and after reaching a certain temperature, the butanone alcohol metering tank 11 starts to add butanone alcohol dropwise. After dropping the heat preservation reaction for a period of time, the reaction kettle and the packing column continue to heat up to a certain temperature. Toluene is used as a water-carrying agent, and the mixed steam of toluene and water is condensed by the condenser 10, and the water is separated through the sight glass, and the toluene is refluxed to the reactor. After reacting for a period of time, stop heating to end the reaction. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com