Preparation method of semaglutide
A liquid phase method and resin technology, which is applied in the field of solid phase synthesis of Samorutai, can solve the problems of unfavorable large-scale production, low purity and yield, and long synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
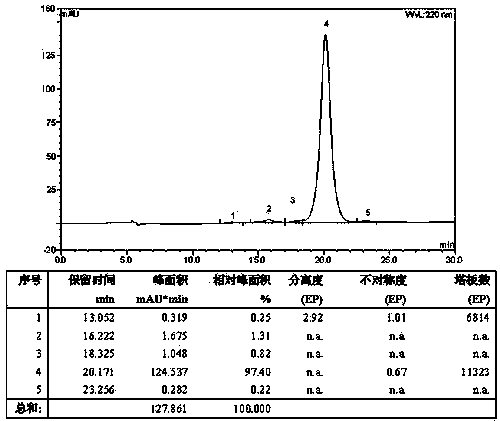
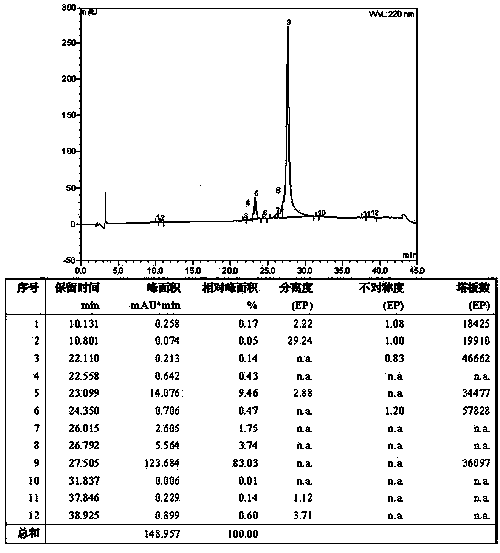
Examples
Embodiment 1
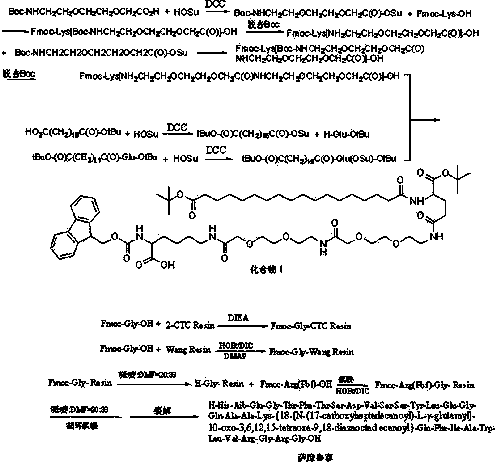
[0099] Embodiment 1: Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 Synthesis of C(O)-OSu Activated Ester
[0100] Weigh 263.29g Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 Add C(O)-OH (1.0mol), 138.10g HOSu (1.2mol) into 2000ml THF, add 247.56g DCC (1.2mol) under ice-water bath, react for 1 hour, raise the temperature to room temperature for 3 hours, and filter the reaction solution. The mother liquor was spin-dried, dissolved in DCM, filtered, washed 3 times with saturated sodium bicarbonate, 2 times with pure water, back-extracted 2 times, combined organic phase, dried with anhydrous sodium carbonate, spin-dried, recrystallized with ice ethanol 3 times, filtered, Solid oil pump dried to obtain 320.72g Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 C(O)-OSu activated ester, yield 89%.
Embodiment 2
[0101] Example 2: Fmoc-Lys[Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 Synthesis of C(O)]-OH
[0102] Weigh 184.21g Fmoc-Lys-OH (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to the mixed solution of 500ml water and 500ml THF to dissolve, and weighed 180.18g Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 Add C(O)-OSu (0.5mol) to 500ml THF, dissolve and add dropwise to the above mixed solution, react overnight at room temperature, adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The resulting white precipitate was recrystallized from ice ethanol. The solid oil pump was dried to obtain 266.96g Fmoc-Lys[Boc-NHCH 2 CH 2 OCH 2 CH 2 OCH 2 C(O)]-OH, yield 87%.
Embodiment 3
[0103] Example three: Fmoc-Lys[NH 2 CH 2 CH 2 OCH 2 CH 2 OCH 2 Synthesis of C(O)]-OH
[0104] Get the 266.96g Fmoc-Lys[Boc-NHCH of above-mentioned embodiment two 2 CH 2 OCH 2 CH 2 OCH 2 C(O)]-OH was added to 500 ml TFA and 500 ml DCM, after adding diethyl ether for precipitation, the solvent was removed by rotary evaporation, and the solid oil pump was dried to obtain 221.23g Fmoc-Lys[NH 2 CH 2 CH 2 OCH 2 CH 2 OCH 2 C(O)]-OH, yield 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com