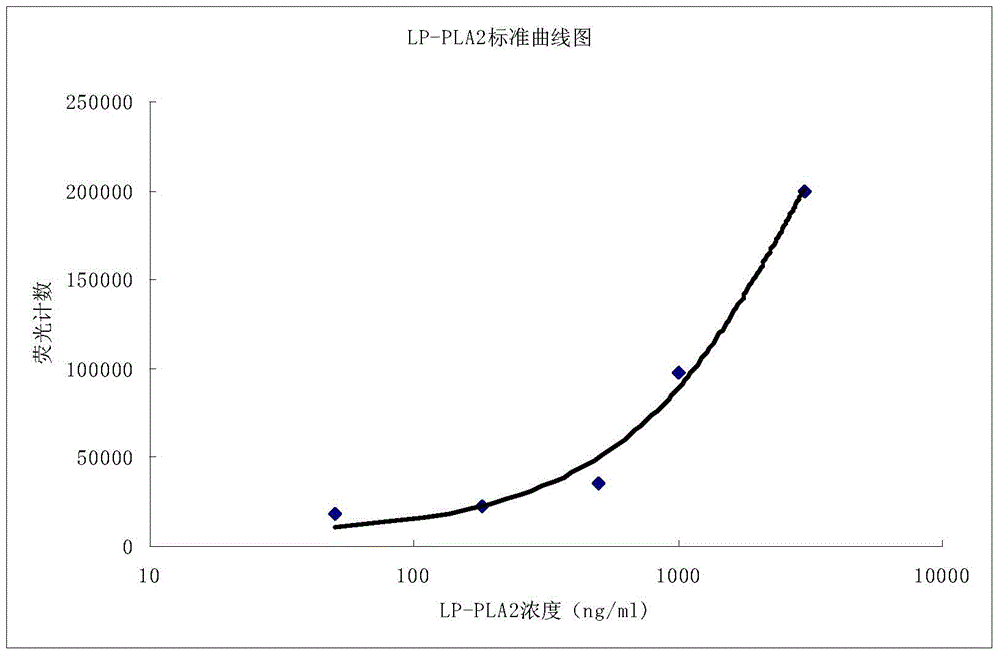
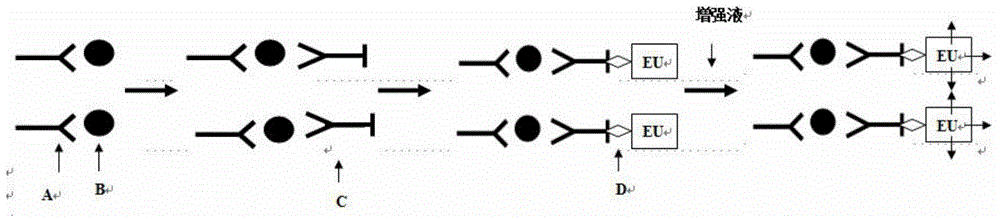
Time-resolved fluorescence immunoassay method of Lp-PLA2 and kit
A technology of time-resolved fluorescence and immunoassay methods, which can be applied in biological testing, measuring devices, and analytical materials, etc. It can solve the problems of time-resolved fluorescence analysis, provision, and lack of kits for Lp-PLA2, and eliminate non-specific Fluorescence interference, wide analysis range, easy automation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Lp-PLA2 time-resolved fluorescence analysis detection kit.
[0034] (1) Preparation of experimental solution:
[0035] Concentrated washing solution: 0.2M phosphate buffered saline (20×PBST) containing 1wt% TWEEN-20;
[0036] Washing solution: 0.01M phosphate buffer containing 0.05wt% TWEEN-20; the washing solution is obtained by diluting the concentrated washing solution;
[0037] Enhancement solution: 1L pH3.2 potassium hydrogen phthalate buffer containing 15 μmol β-naphthoyltrichloroacetone (β-NTA), 50 μmol tri-n-octylphosphine oxide TOPO and 1 mL Triton X-100 (Triton X100) .
[0038] (2) Preparation and purification of Lp-PLA2 monoclonal antibody A and Lp-PLA2 monoclonal antibody B:
[0039]Lp-PLA2 monoclonal antibody A and Lp-PLA2 monoclonal antibody B recognize different epitopes of Lp-PLA2 respectively and do not affect each other. Among them, Lp-PLA2 monoclonal antibody A and B can be obtained by monoclonal hybridoma cell technology ...
Embodiment 2
[0053] Example 2 The precision, accuracy and stability test of the Lp-PLA2 time-resolved fluorescence analysis detection kit.
[0054] 1. The accuracy and precision analysis experiment of the kit:
[0055] The standard solution of 207ng / ml Lp-PLA2 (pH7.4, 0.05M phosphate buffer) was tested 20 times. 207ng / ml is the critical value of Lp-PLA2 to predict the high risk of cardiovascular and cerebrovascular diseases. The high accuracy of the method is conducive to the accurate judgment of the risk of disease. The average value of 20 test results was 204.64ng / ml, the standard deviation was 8.26, and the variation rate was 4.03%. The results show that the detection result is close to the actual concentration, and the accuracy is high, and the variation rate is less than 5%, which has good accuracy.
[0056] 2. Kit stability test:
[0057] The storage condition of the kit is 2-8°C. After 6 months of storage, various indicators of the kit were measured and found to be within the nor...
Embodiment 3
[0058] Example 3 Comparison of Lp-PLA2 Time-Resolved Fluorescence Analysis Detection Kit and ELISA Detection Kit.
[0059] Select Lp-PLA2 standard solution with three concentrations of high, medium and low, and analyze it with this kit and ELISA detection kit at the same time. Each concentration is tested 5 times, and the results are compared to determine the consistency of the two methods. . Choose 1000ng / ml for high concentration, 300ng / ml for medium concentration, and 80ng / ml for low concentration. The test results obtained are as follows:
[0060]
[0061] The t-test of paired samples was performed on the two groups of data, and the result was P>0.05, and the difference between the two was not statistically significant, indicating that the two methods had good consistency in the test of high, medium or low concentration of Lp-PLA2 sex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com