Novel pyruvate kinase M2 activator and its synthetic method
A technology of pyruvate kinase and synthesis method, which is applied in the field of new pyruvate kinase M2 activator and its synthesis, can solve problems such as complex process, and achieve the effect of reducing synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
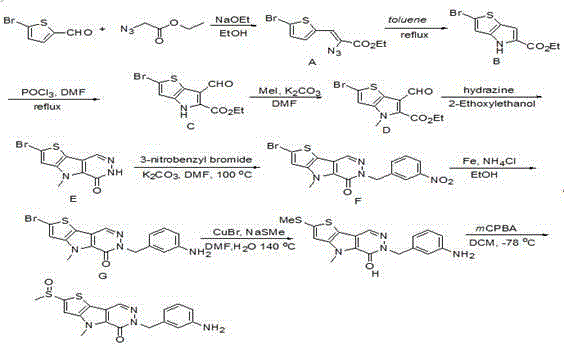
[0030] Step 1) Sodium (2.76 g, 120 mmol) was added portionwise to anhydrous EtOH (120 mL). After the sodium was completely dissolved, the solution was cooled to -10°C in an ice-salt bath, and 5-bromo-2-formylthiophene (5.73 g, 30 mmol) and ethyl azidoacetate (10.1 g, 60 mmol mol) mixture, added dropwise to the reaction system within 30 minutes. Stir at this temperature for one hour, then react at 0°C for one hour, remove the ice bath, and stir the reaction mixture at room temperature for another 30 minutes. TLC monitored the completion of the reaction. The reaction solution was added with saturated aqueous ammonium chloride solution (100 ml), and then poured into 5 times the volume of cold water, stirred, and solids were precipitated. After filtration, the solid was washed three times with water. As a light yellow solid, this solid was used directly in the next step.
[0031] Step 2) Dissolve the light yellow powder from the previous step in toluene (50 ml), dry it with an...
Embodiment 2
[0041] Step 1) Sodium (27.6 g, 1.2 mol) was added in portions to anhydrous EtOH (1.2 L). After the sodium was completely dissolved, the solution was cooled to -10°C in an ice-salt bath, and 5-bromo-2-formylthiophene (57.3 g, 300 mmol) and ethyl azidoacetate (101 g, 600 mmol mol) mixture, added dropwise to the reaction system within 30 minutes. Stir at this temperature for one hour, then react at 0°C for one hour, remove the ice bath, and stir the reaction mixture at room temperature for another 30 minutes. TLC monitored the completion of the reaction. Add saturated ammonium chloride aqueous solution (1 liter) to the reaction solution, then pour it into 5 times the volume of cold water, stir, and solid precipitates out. After filtration, the solid was washed three times with water. As a light yellow solid, this solid was used directly in the next step.
[0042] Step 2) Dissolve the light yellow powder from the previous step in toluene (500 ml), dry with anhydrous sodium sul...
Embodiment 3
[0052] Step 1) Sodium (55 g, 2.4 mol) was added portionwise to anhydrous EtOH (2.4 L). After the sodium was completely dissolved, the solution was cooled to -10°C in an ice-salt bath, and 5-bromo-2-formylthiophene (114.6 g, 600 mmol) and ethyl azidoacetate (200 g, 1.2 mole ) mixture was added dropwise to the reaction system within 30 minutes. Stir at this temperature for one hour, then react at 0°C for one hour, remove the ice bath, and stir the reaction mixture at room temperature for another 30 minutes. TLC monitored the completion of the reaction. Add saturated ammonium chloride aqueous solution (2 liters) to the reaction solution, then pour it into 5 times the volume of cold water, stir, and solid precipitates out. After filtration, the solid was washed three times with water. As a light yellow solid, this solid was used directly in the next step.
[0053] Step 2) Dissolve the light yellow solid powder in the previous step in toluene (1 liter), dry it with anhydrous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com