Antimicrobial peptide AP-57 as well as preparation method and application method thereof
An AP-57, application method technology, applied in the fields of biochemistry and biomedicine, can solve the problems of few types of human antimicrobial peptides, difficult production and quality control, and small number of amino acids, and achieves significant antibacterial and antifungal, obvious antibacterial and antifungal properties. Anti-enveloped virus and mycoplasma, the effect of large development potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
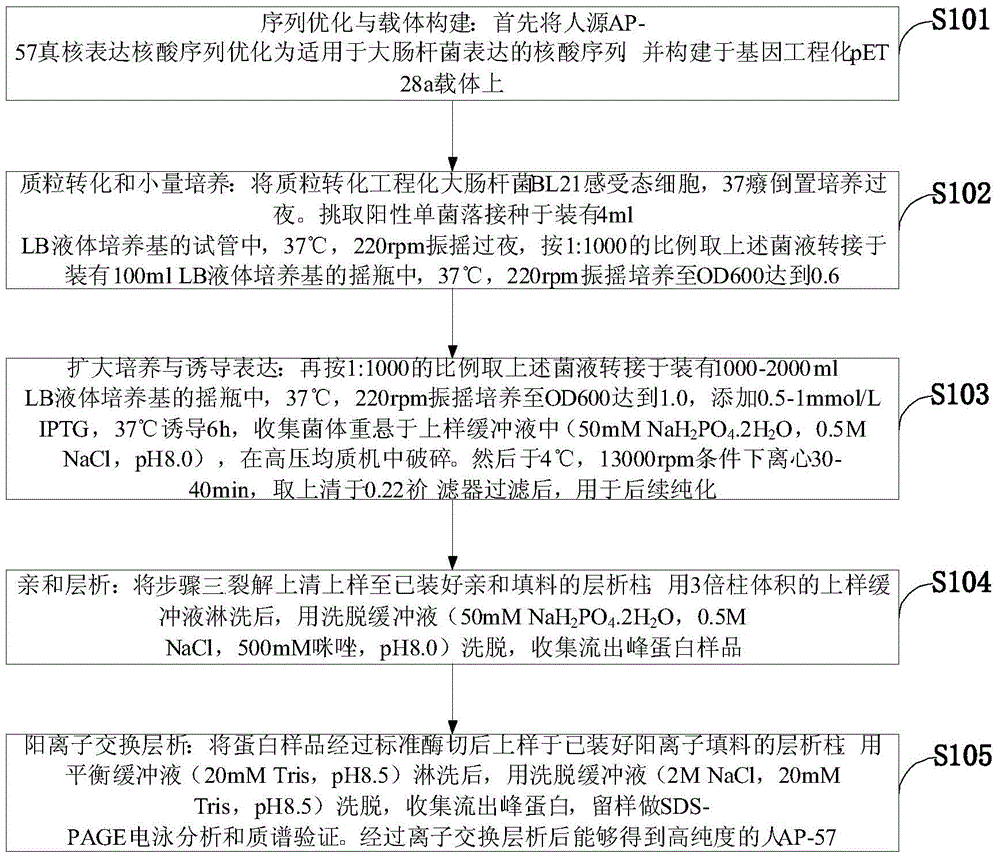
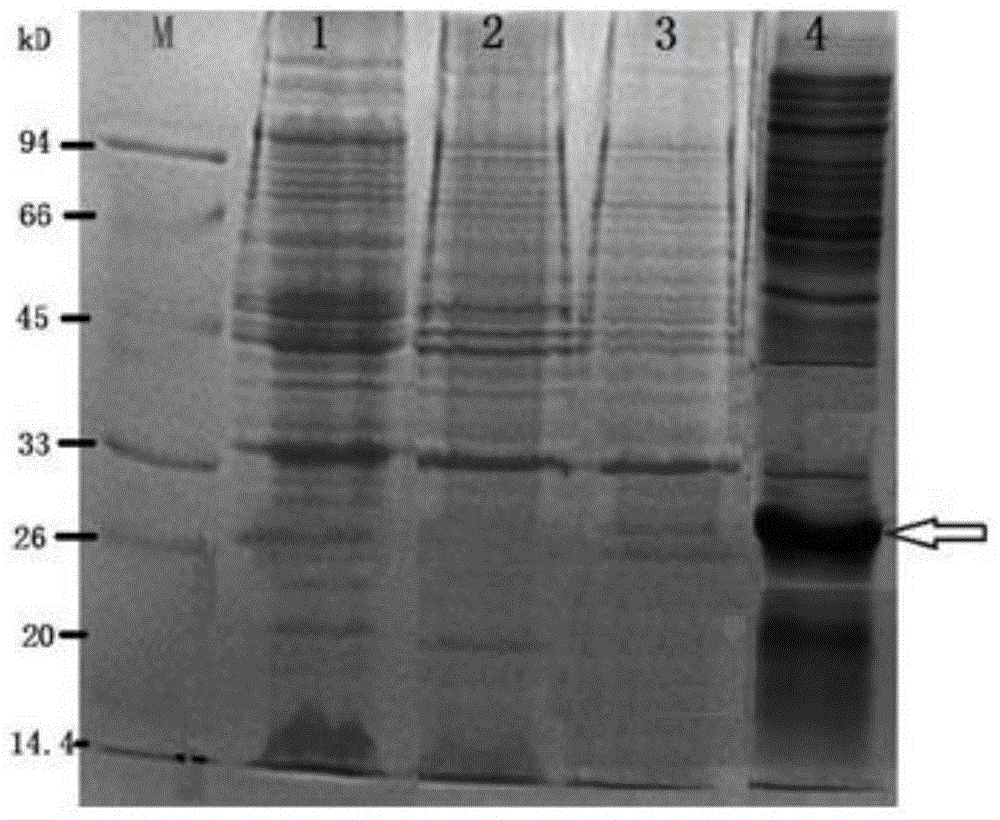
[0043] In order to make the object, technical solution and advantages of the present invention more clear, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0044] The application principle of the present invention will be further described below in conjunction with the accompanying drawings and specific embodiments.
[0045] The antibacterial peptide AP-57 of the embodiment of the present invention is a polypeptide, corresponding to the mature protein sequence of the C10orf99 gene expression product after removing the signal peptide, containing 57 amino acids, and the sequence is:
[0046] KRRPAKAWSGRRTRLLCCHRVPSPNSTNLKGHHVRLCKPCKLEPEPRLWVVPGALPQV;
[0047] Theoretical molecular weight (MW) is: 6.52D; theoretical isoelectric point (pI) is 11.28; it is a strong cationic amphip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com