Method for preparing benzophenone through biomimetic catalytic oxidation
A technology of benzophenone and biomimetic catalysis, which is applied in the direction of oxidative preparation of carbonyl compounds, chemical instruments and methods, catalytic reactions, etc., can solve the problems of low efficiency, high cost, harsh conditions, etc., and achieve high efficiency, low energy consumption, The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
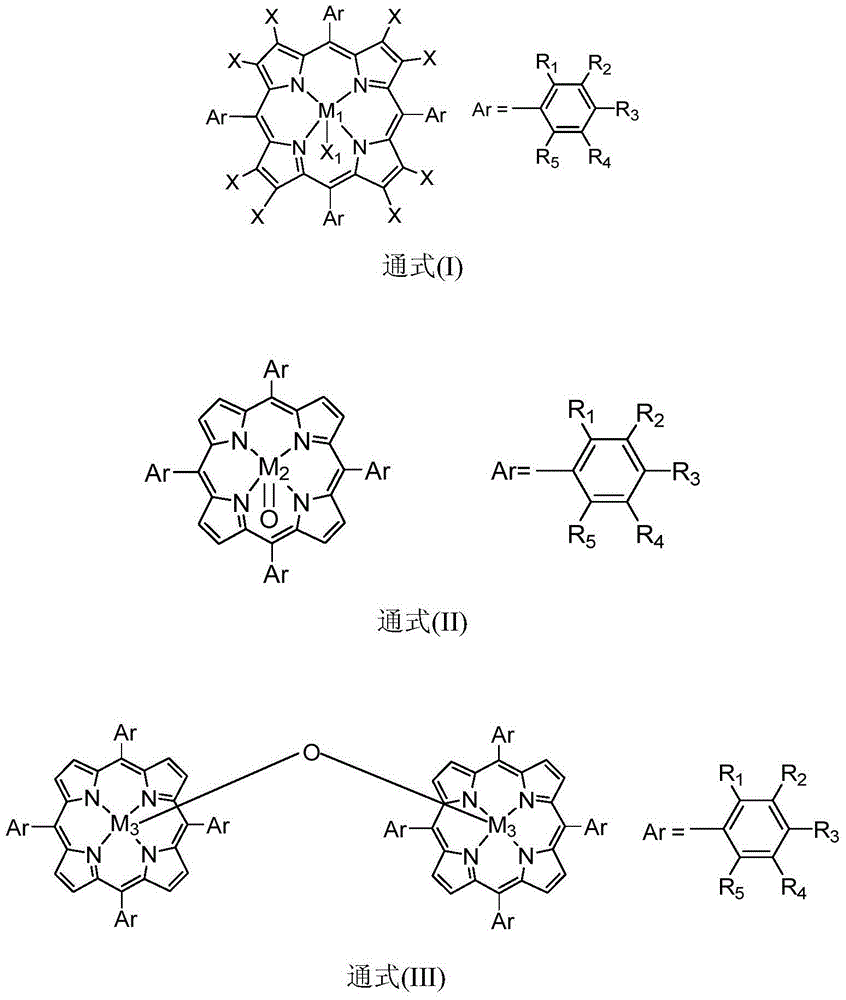
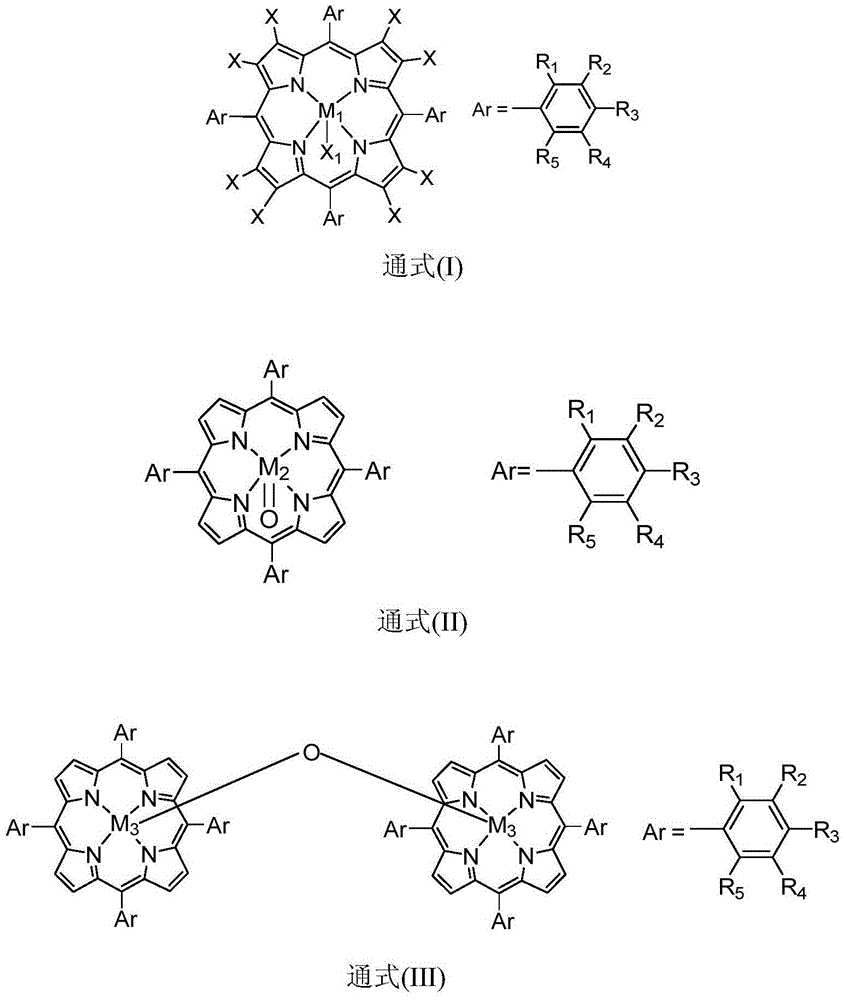
[0019] Containing 5ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Mn,X=H,R 1 =R 2 =R 3 =R 4 =R 5 =H,X 1 =Cl) in the hexanaphthene solution, add the tert-butyl hydroperoxide of 2mmol diphenylmethane and 2mmol, at temperature be 40 ℃ of stirring reaction 4 hours, through detection and analysis, the conversion rate of diphenylmethane is 82%, The selectivity of benzophenone is greater than 99%.
Embodiment 2
[0021] Contain 100ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Co,X=H,R 1 = NO 2 , R 2 =R 3 =R 4 =R 5 =H,X 1 In the trifluorotoluene solution of pyridine), add 2mmol of diphenylmethane and 4mmol of hydrogen peroxide, and stir and react at a temperature of 100° C. for 6 hours. After detection and analysis, the conversion rate of diphenylmethane is 83%, and diphenylmethane Ketone selectivity is greater than 99%.
Embodiment 3
[0023] Contain 60ppm metalloporphyrin (M) with general formula (I) structure in 10mL 1 =Cr,X=F,R 1 =R 2 =R 3 =R 4 =R 5 =H,X 1 In the sec-butyl acetate solution of imidazole), add 2 mmol of diphenylmethane and 1 mmol of m-chloroperoxybenzoic acid, and stir and react at a temperature of 80° C. for 8 hours. After detection and analysis, the conversion rate of diphenylmethane is 93%. , The selectivity of benzophenone is greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com