Preparation method of bis(methylenedioxy) biphenyl derivative compound
A methylenedioxy compound technology, which is applied in the field of preparation of biphenyl derivative compounds, can solve problems such as inappropriate process safety, and achieve the effects of simplifying the production process and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
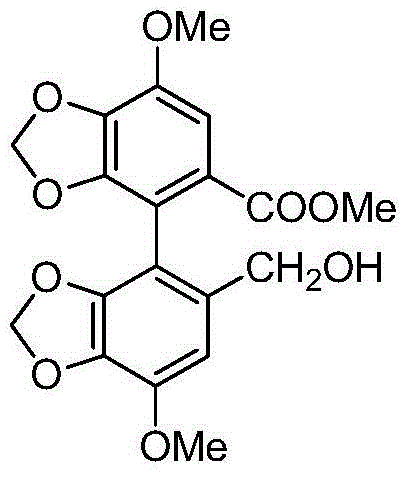
[0039] The structural formula of the bis(methylenedioxy)biphenyl derivative compound formula I in this example is as follows:
[0040]
[0041] In formula I, R ' and R " are methyl, and the synthetic method of this compound is as follows:
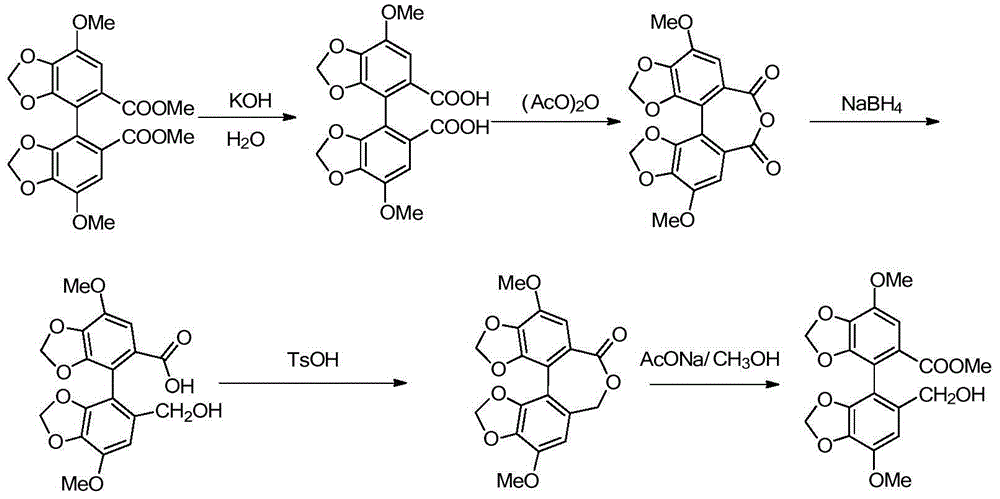
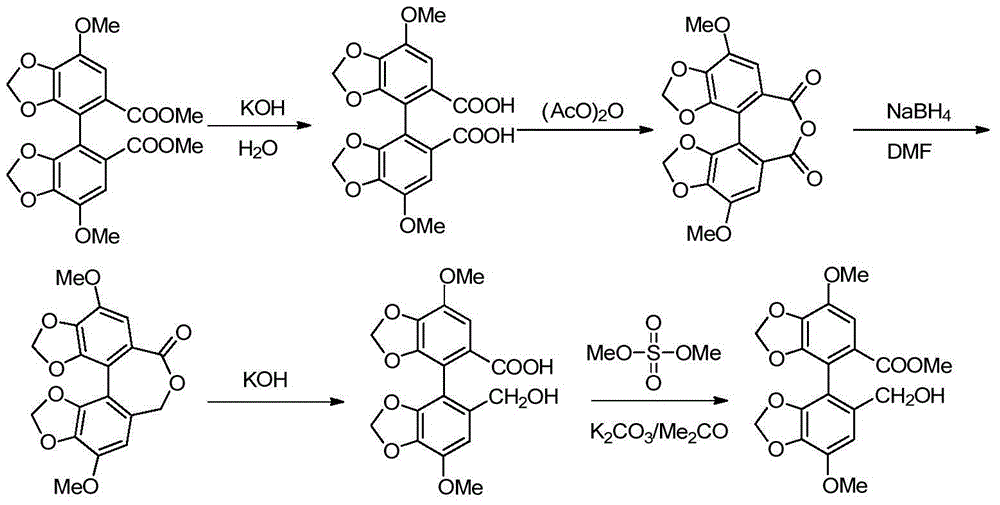
[0042] In a 500mL autoclave, 37.2g (0.1mol) of biphenyl anhydride (that is, R" in the compound of formula II is a methyl group), 1.9g (0.05mol) of sodium borohydride, 0.25mol of glacial acetic acid or trifluoroacetic acid, methanol solvent 300mL, mix uniformly at 0-10°C, replace with nitrogen, seal the reaction system, then slowly raise the temperature to 50°C-60°C and stir for 6 hours, then raise the temperature to 100°C-150°C and stir for 5 hours, the reaction is over After cooling to room temperature, the reaction solution was exported out of the autoclave, quenched, filtered, concentrated to remove the solvent, then added saturated aqueous sodium hydroxide solution to adjust the pH value of the system to 8-9 for alkalization and wash...
Embodiment 2
[0044] The structural formula of the bis(methylenedioxy)biphenyl derivative compound formula I in this example is as follows:
[0045]
[0046] In formula I, R ' and R " are ethyl, and the synthetic method of this compound is as follows:
[0047] In a 500mL autoclave, 40g (0.1mol) of biphenyl anhydride (that is, R" in the compound of formula II is ethyl), 3.8g (0.1mol) of sodium borohydride, 0.4mol of glacial acetic acid or trifluoroacetic acid, and 300mL of ethanol solvent , mix uniformly under the condition of 0-10°C, replace with nitrogen, seal the reaction system, then slowly raise the temperature to 50°C-60°C and stir for 5 hours, then raise the temperature to 100°C-150°C and stir for 4 hours, after the reaction , cooled to room temperature, the reaction solution was exported to the autoclave, quenched, filtered, concentrated to remove the solvent, and then added saturated aqueous sodium hydroxide solution to adjust the pH value of the system to 8-9 for alkaline washin...
Embodiment 3
[0049] The structural formula of the bis(methylenedioxy)biphenyl derivative compound formula I in this example is as follows:
[0050]
[0051] In formula I, R' is methyl, R " is phenyl, and the synthetic method of this compound is as follows:
[0052] In a 500mL autoclave, 49.6g (0.1mol) of biphenyl anhydride (that is, R" in the compound of formula II is phenyl), 7.6g (0.2mol) of sodium borohydride, 0.6mol of glacial acetic acid or trifluoroacetic acid, methanol solvent 300mL, mix uniformly under the condition of 0-10°C, replace with nitrogen, seal the reaction system, then slowly raise the temperature to 55°C for stirring reaction for 6 hours, then raise the temperature to 120°C and stir for 5 hours, after the reaction is completed, cool to room temperature , the reaction solution is exported to the autoclave, quenched, filtered, concentrated to remove the solvent, and then add saturated aqueous sodium bicarbonate to adjust the pH value of the system to 8-9 for alkaline w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com