Method for simply and massively preparing graphene dispersed molybdenum base sulfide catalyst
A graphene and sulfide technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Low-cost, wide-ranging, and simple-to-method effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Seal 60g of ball milling balls (stainless steel balls, 1.2cm in diameter), 0.32g of graphene, 0.68g of ammonium tetrathiomolybdate, and 20ml of hydrazine hydrate into a ball milling tank under argon protection.
[0033] 2. Put the ball mill jar in (1) on a ball mill and mill it at a speed of 450 rpm for 20 hours.
[0034] 3. Take out the ball milling ball with tweezers, filter the sample with suction, and dry the sample in an oven at 100°C to obtain the catalyst.
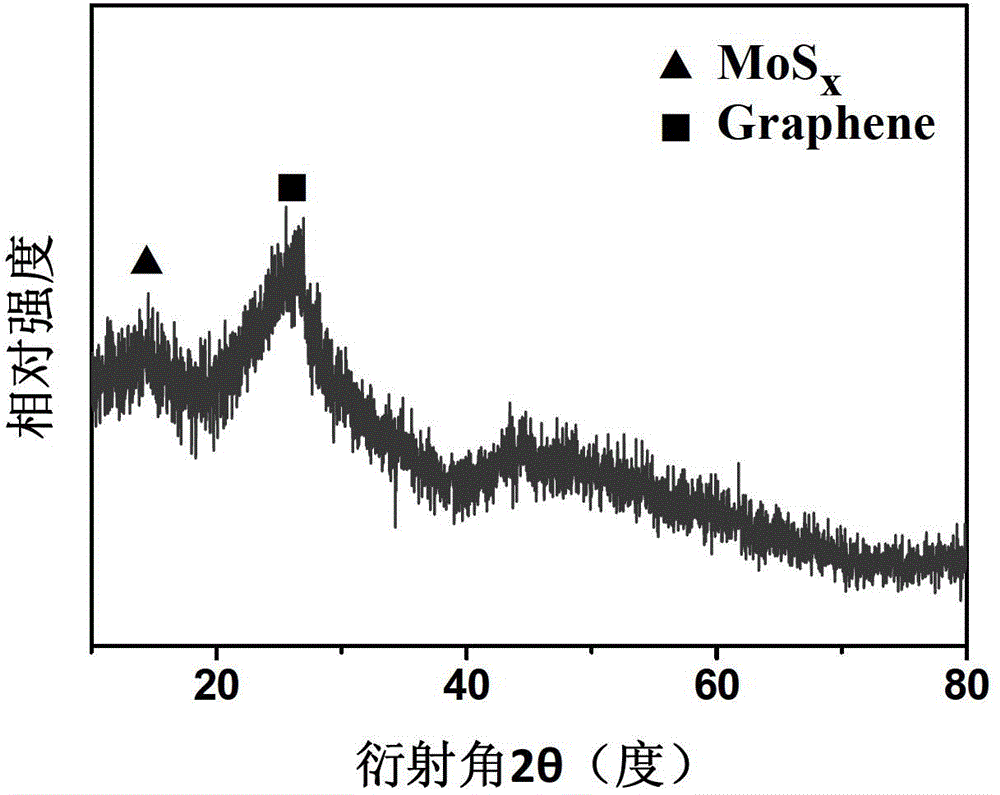
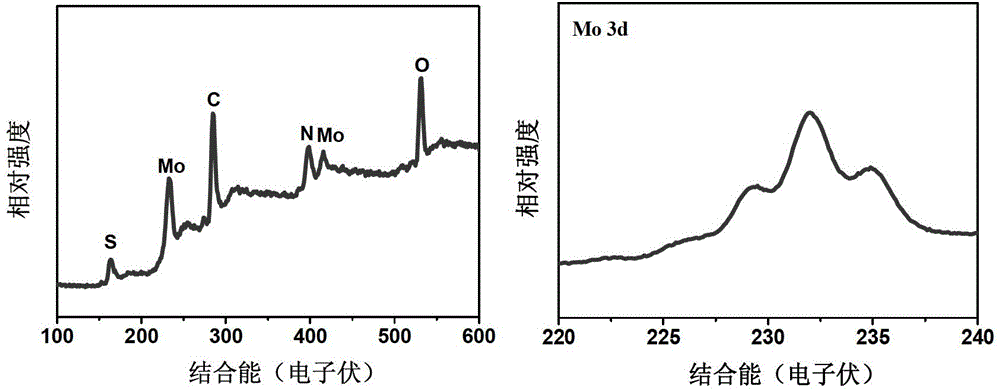
[0035] 0.65g of catalyst can be prepared in one time. X-ray diffraction spectrum (see figure 1 ) shows that the diffraction peak distribution of the molybdenum-based sulfide in the obtained catalyst is wider, indicating that the obtained molybdenum-based sulfide is more dispersed. X-ray photoelectron spectroscopy (see figure 2 ) shows that the prepared catalyst contains mainly C, N, O, S, Mo, and the valence state of Mo element is +4 valence and +6 valence.
Embodiment 2
[0037] 1. Seal 60g of ball milling balls (zirconia balls, 0.2cm in diameter), 0.32g of nitrogen-doped graphene, 0.68g of ammonium tetrathiomolybdate, and 10ml of hydrazine hydrate into a ball milling jar under argon protection.
[0038] 2. Put the ball mill jar in (1) on a ball mill and mill at a speed of 500 rpm for 10 hours.
[0039] 3. Separate the balls from the sample through a sieve, filter the sample with suction, and dry the sample in an oven at 120°C to obtain the catalyst.
[0040] About 0.6g of catalyst can be prepared in a single tank. The X-ray diffraction spectrum shows that the molybdenum-based sulfides in the obtained catalyst are relatively dispersed. X-ray photoelectron spectroscopy showed that the valence state of Mo element in the prepared catalyst was +4 and +6.
Embodiment 3
[0042] 1. Seal 180g of ball milling balls (zirconia balls, 0.8cm in diameter), 0.64g of boron-doped graphene, 1.36g of ammonium polythiomolybdate, and 5ml of ammonia water into a ball milling jar under nitrogen protection.
[0043] 2. Put the ball mill jar in (1) on a ball mill and mill at a speed of 600 rpm for 10 hours.
[0044] 3. Separate the ball milling balls from the sample through a sieve, and filter with suction. The catalyst was obtained by drying the sample in an oven at 150°C.
[0045] About 1.5g of catalyst can be prepared in a single tank. The X-ray diffraction spectrum shows that the molybdenum-based sulfides in the obtained catalyst are relatively dispersed. X-ray photoelectron spectroscopy showed that the valence state of Mo element in the prepared catalyst was +4 and +6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com