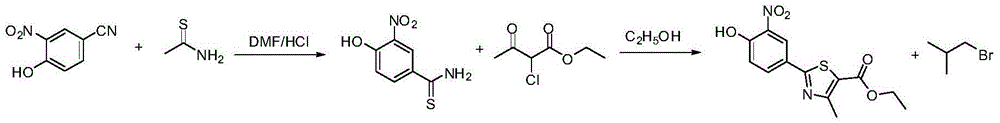
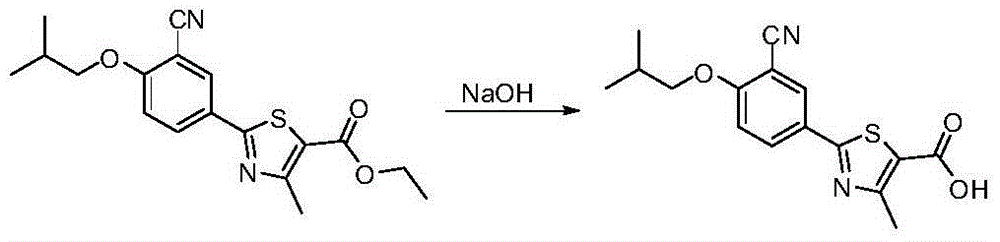
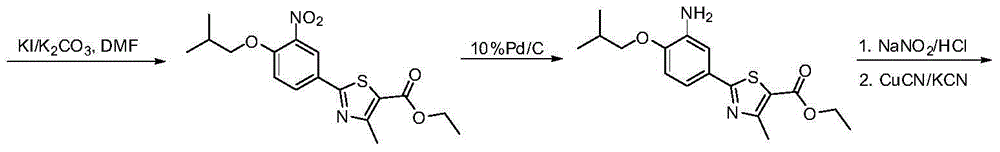Preparation method of febuxostat intermediate
A febuxostat and intermediate technology, applied in the field of medicine, can solve the problems of harsh reaction conditions, strong corrosion, and highly toxic reaction raw materials, and achieve the effect of mild reaction conditions, low toxicity, and low corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 p-hydroxyl thiobenzamide
[0034] Add 1.5g of p-cyanophenol and 15mL of DMF into a 250mL three-necked flask, stir, then add 2.02g of 70% sodium hydrosulfide and 3.84g of magnesium chloride hexahydrate, and react the reaction mixture at 25 degrees for 24 hours, TLC detection (dichloromethane: Methanol=30:1, volume ratio) The raw materials basically reacted completely. Cool, filter with suction, pour into stirred ice water (100mL), extract the aqueous phase with ethyl acetate (3×30mL), combine the organic phases and dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure to obtain 1.5g of a brown solid, yield 90%.
Embodiment 22
[0035] Synthesis of Example 22-(4-hydroxyphenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester
[0036] Add 0.7g of p-hydroxythiobenzamide, 2.8mL of ethanol and 0.9g of ethyl 2-chloroacetoacetate into a 50mL three-necked flask, heat the reaction mixture under reflux for 2 hours, and detect it by TLC (dichloromethane:methanol=30:1 , volume ratio) the raw materials basically reacted completely. Cool, filter with suction, rinse the filter cake with ethanol, and dry the filter cake in vacuum at 50°C to obtain 1.0 g of a yellow solid with a yield of 84%.
Embodiment 3
[0037] Synthesis of Example 32-(3-formyl-4-hydroxyphenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester
[0038] Add 2.0g (0.0076mol) of ethyl 2-(4-hydroxyphenyl)-4-methyl-thiazole-5carboxylate and 20mL glacial acetic acid into a 50mL three-necked flask, heat up to 80-90°C, and then add 2.1 g (0.015mol) urotropine, 1.0g (0.0027mol) cerium trichloride, the reaction mixture was heated to 118°C for 4 hours, TLC detection (petroleum ether: ethyl acetate = 3:1, volume ratio) raw materials The basic response is complete. Cool to room temperature, add 0.05mL of concentrated sulfuric acid dropwise, add 20mL of water after 5 minutes, shake fully, a large amount of solid precipitates, extract with ethyl acetate (3×30mL), combine the organic phases and wash with saturated brine until neutral, the organic phase is free of Water was dried over sodium sulfate, and the solvent was distilled off under reduced pressure to obtain 1.0 g of a yellow solid with a yield of 50% and a HPLC purity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com