Carbazolyl blue phosphorescent host material as well as preparation method and application thereof
A phosphorescent host and host material technology, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as shortage, and achieve the effects of reduced manufacturing cost, simple synthesis route, and high hole mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
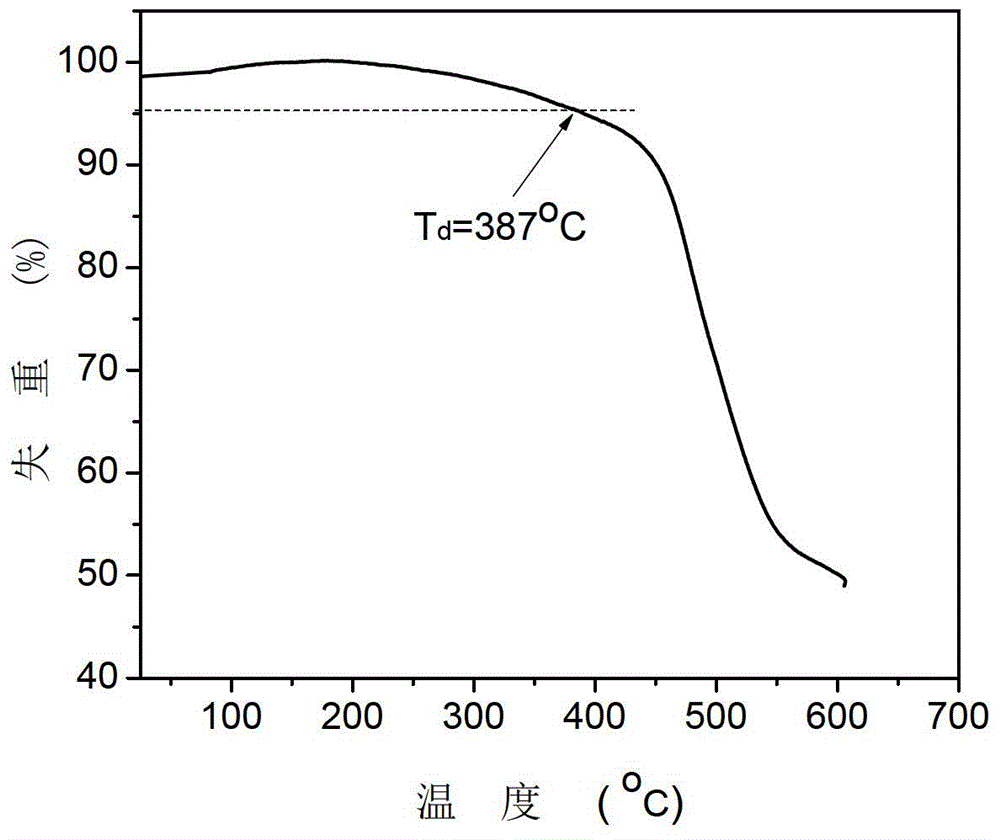
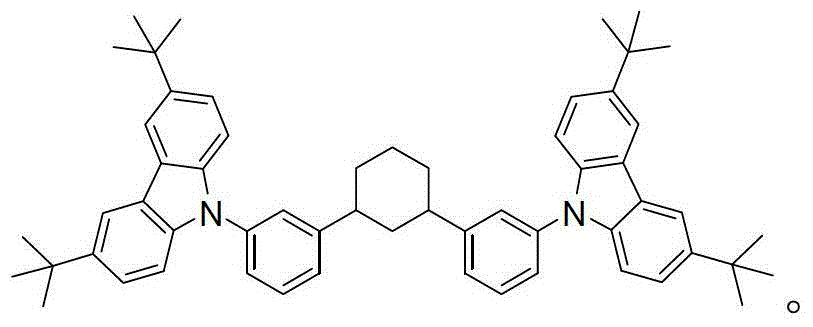
[0025] Example 1: The carbazolyl blue phosphorescent host material of this example, namely 1,3-bis(3-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)cyclohexane , the structural formula is as follows:
[0026]
[0027] The preparation process of this compound is as follows:
[0028]
[0029] Under argon protection, 1,3-dibromocyclohexane (48 mg, 0.2 mmol), 3,6-di-tert-butyl-9-(3-pinacol borate phenyl)-9H-carbazole (192mg, 0.4mmol) was added to a flask containing 10ml of toluene solvent, and after fully dissolving, potassium carbonate (2mL, 2mol / L) solution was added to the flask, vacuumed to remove oxygen and filled with argon, and then added bistriphenyl Phosphine palladium dichloride (5.6mg, 0.008mmol); the flask was heated to 120°C for Suzuki coupling reaction for 24h. Stop the reaction and cool to room temperature, combine the organic phases after extracting with dichloromethane several times, then dry the organic phases with anhydrous magnesium sulfate and spin dry to ...
Embodiment 2
[0031] Example 2: The carbazolyl blue phosphorescent host material of this example, namely 1,3-bis(3-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)cyclohexane , the structural formula is as follows:
[0032]
[0033] The preparation process of this compound is as follows:
[0034]
[0035] Under the protection of mixed gas of nitrogen and argon, 1,3-dibromocyclohexane (73 mg, 0.3 mmol), 3,6-di-tert-butyl-9-(3-pinacol borate phenyl)- 9H-carbazole (317mg, 0.66mmol) and 15mL tetrahydrofuran were added into a 50mL two-necked bottle, fully dissolved, and then a mixture of nitrogen and argon was introduced to exhaust the air for about 20 minutes, and tetrakistriphenylphosphine palladium (4mg, 0.003mmol) into it, fully dissolved and then added sodium bicarbonate (3mL, 2mol / L) solution. Then, the mixed gas of nitrogen and argon was exhausted for about 10 minutes, and the two-neck flask was added to 70°C for Suzuki coupling reaction for 48 hours. Stop the reaction and cool to roo...
Embodiment 3
[0036] Example 3: The carbazolyl blue phosphorescent host material of this example, namely 1,3-bis(3-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)cyclohexane , the structural formula is as follows:
[0037]
[0038] The preparation process of this compound is as follows:
[0039]
[0040] Under nitrogen protection, 1,3-dibromocyclohexane (73 mg, 0.3 mmol), 3,6-di-tert-butyl-9-(3-pinacol borate phenyl)-9H-carbazole ( 346mg, 0.72mmol), palladium acetate (3.5mg, 0.015mmol) and three (o-methoxyphenyl) phosphine (21mg, 0.06mmol) were added to the flask containing 12mL of N,N-dimethylformamide , after fully dissolving, add potassium carbonate (3mL, 2mol / L) solution, then blow nitrogen into the flask and exhaust the air for about 30min; heat the flask to 130°C for Suzuki coupling reaction for 12h. Stop the reaction and cool to room temperature, combine the organic phases after extracting with dichloromethane several times, then dry the organic phases with anhydrous magnesium su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com