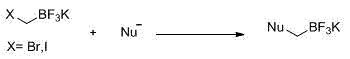Preparation process of (bromomethyl) trifluoro potassium borate
A technology of potassium trifluoroborate and preparation process, which is applied in the fields of compounds containing elements of Group 3/13 of the periodic table, chemical instruments and methods, organic chemistry, etc. problem, to achieve the effect of low cost, simple operation and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation technique for (bromomethyl) potassium trifluoroborate, comprising the following steps:
[0025] 1) Add n-butyllithium, azaborane and organic solvent into the reaction vessel;
[0026] 2) Add dibromomethane dropwise within 1.5-3.5 hours to react, and control the temperature of the system at -20°C-30°C;
[0027] 3) Add KHF at 20-30°C 2 The aqueous solution is reacted at 80-100°C for 6-10h;
[0028] 4) Separation and purification of the reaction system in the previous step to obtain the product.
[0029] The structural formula of described nitrogen borane is as follows:
[0030] ; ; ; ;
[0031] Among them, R 1 , R 2 , R 3 , R 4 For independent protecting group, X is chlorine or bromine, n=1~5.
[0032] The protecting group (i.e. R 1 , R 2 , R 3 , R 4 ) is selected from the following groups: methyl, ethyl, n-propyl, isopropyl, allyl, n-butyl, isobutyl, tert-butyl, 2-enyl, 3-enyl, 2 -Methyl-2-allyl, n-pentyl, 1-methylbutyl, 2-methylbutyl,...
Embodiment 1
[0040] In a dry 500 mL four-necked reaction flask, add 80 mL of 2.5M / L n-butyllithium, 27 g of bis(dimethylamino)chloroborane ( ) and 200 mL THF were added to the reaction flask. Then start to drop 1.0 g of dibromomethane at room temperature, and stir for half an hour. Continue to drop 28 g of dibromomethane for 2 hours, and control the temperature of the reaction solution at 15°C. Then add 33 g KHF at room temperature 2100 mL of the aqueous solution, the temperature is controlled at 80-100 degrees and the stirring is continued for 8 hours. After the reaction was monitored by TLC, the reaction solution was concentrated under reduced pressure. The residue was dissolved and filtered with 120 mL of acetone, and the mother liquor was concentrated under reduced pressure. The resulting residue was slurried with 100 mL of methyl tert-butyl ether and filtered. The wet product was vacuum-dried to obtain 15.6 g (bromomethyl) potassium trifluoroborate with a yield of 70%. The char...
Embodiment 2
[0042] In a dry 500 mL four-necked reaction flask, 80 mL of 2.5M / L n-butyllithium, 27 g of bis(dimethylamino)chloroborane ( ) and 200 mL of ether were added to the reaction flask. Then start to drop 1.0 g of dibromomethane at room temperature, and stir for half an hour. Continue to drop 28 g of dibromomethane for 2 hours, and control the temperature of the reaction solution at 10°C. Then add 33 g KHF at room temperature 2 100 mL of aqueous solution and 100 mL of tetrahydrofuran, and the temperature was controlled at 80-100 degrees to continue stirring for 8 hours. After the reaction was monitored by TLC, the reaction solution was concentrated under reduced pressure. The residue was dissolved and filtered with 120 mL of acetone, and the mother liquor was concentrated under reduced pressure. The resulting residue was slurried with 100 mL of methyl tert-butyl ether and filtered. The wet product was vacuum-dried to obtain 14.8 g (bromomethyl) potassium trifluoroborate with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com