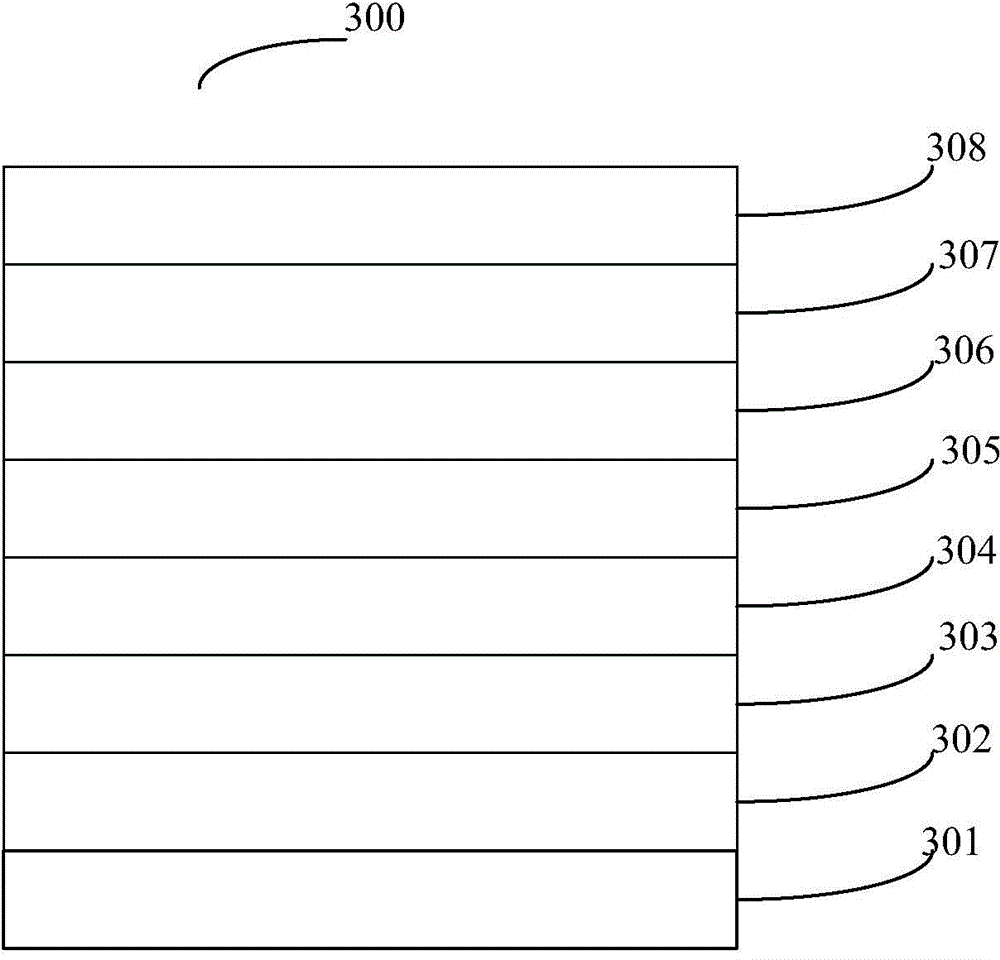
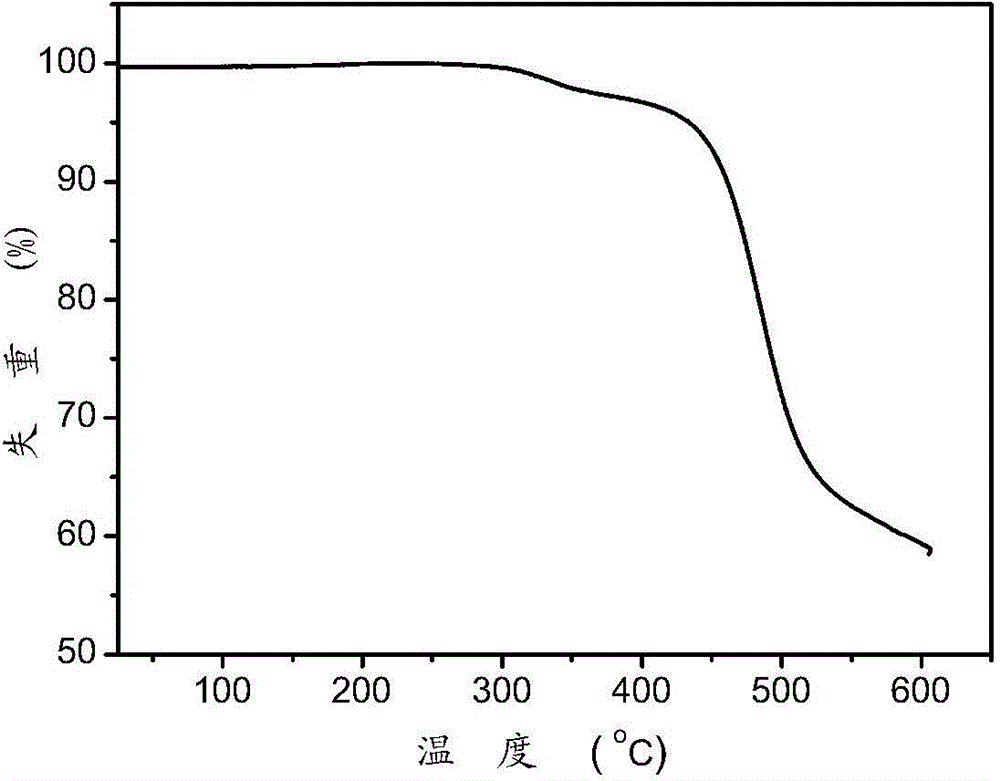
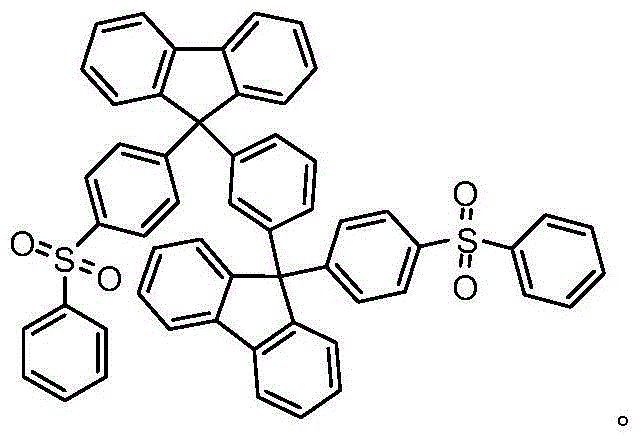
Organic semiconductor material, preparation method thereof and electroluminescent device
An electroluminescent device and organic semiconductor technology, applied in the fields of organic semiconductor materials, electroluminescent devices, and preparation, can solve problems such as lack of host materials and few red phosphorescent devices, improve luminous efficiency, and facilitate carriers The effect of transmission balance, simple and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of organic semiconductor material, comprises the steps:
[0027] Provides compound A: and compound B: Under an inert atmosphere, first dissolve compound A into an organic solvent to form a solution with a concentration of 0.3mol / L to 0.5mol / L, then add compound B, an inorganic base and a catalyst to the reactant at 70 to 120°C The reaction was carried out for 6 to 15 hours, the molar ratio of compound A to compound B was 1:2 to 1:2.4, the reaction was stopped and cooled to room temperature, filtered and washed with water to obtain an off-white solid, the crude product was washed with n-hexane as eluent Silica gel chromatography column separation obtains the chemical formula of the intermediate product as follows:
[0028]
[0029] Dissolve the intermediate product in the second solvent at zero degrees Celsius, then drop m-chlorobenzoic acid into the second solvent, react at room temperature for 8 hours to 16 hou...
Embodiment 1
[0042] The preparation process of 1,3-bis(9-(4-(benzenesulfonyl)phenyl)-9H-fluoren-9-yl)benzene The preparation steps are as follows:
[0043]
[0044] Under nitrogen protection, 1,3-bis(9-(4-bromophenyl)-9H-fluorene-9-yl)benzene (57.3g, 80mmol) was dissolved in 200mL N,N-dimethylformamide (DMF ) solution, and then added thiophenol (17.6g, 160mmol), potassium carbonate (22.1g, 160mmol) and cuprous iodide (1.52g, 8mmol). The reactants were stirred and reacted at 120° C. for 6 hours. Stop the reaction and cool to room temperature, filter, and wash the solid three times with distilled water. The crude product is separated by silica gel column chromatography using n-hexane as eluent to obtain an intermediate product. The yield was 84%.
[0045] Under ice-cooling, the intermediate product (30.9 g, 40 mmol) was dissolved in 120 mL of dichloromethane (DCM) solution, and then it was dropped into 90 mL of m-chlorobenzoic acid (mCPBA) in dichloromethane solution. After the mixture...
Embodiment 2
[0051] The preparation process of 1,3-bis(9-(4-(benzenesulfonyl)phenyl)-9H-fluoren-9-yl)benzene The preparation steps are as follows:
[0052]
[0053] Under nitrogen protection, 1,3-bis(9-(4-bromophenyl)-9H-fluorene-9-yl)benzene (57.3g, 80mmol) was dissolved in 267mL of toluene (Tol) solution, and then added benzene Thiophenol (19.4g, 176mmol), cesium carbonate (57.2g, 176mmol), copper powder (0.768g, 12mmol). The reactants were stirred and reacted at 110° C. for 9 hours. Stop the reaction and cool to room temperature, filter, and wash the solid three times with distilled water. The crude product is separated by silica gel column chromatography using n-hexane as eluent to obtain an intermediate product. The yield was 85%.
[0054] Under ice-cooling, the intermediate product (30.9 g, 40 mmol) was dissolved in 120 mL of chloroform solution, and then it was dropped into 90 mL of m-chlorobenzoic acid (mCPBA) in dichloromethane solution. After the mixture was stirred and rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electron mobility | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com