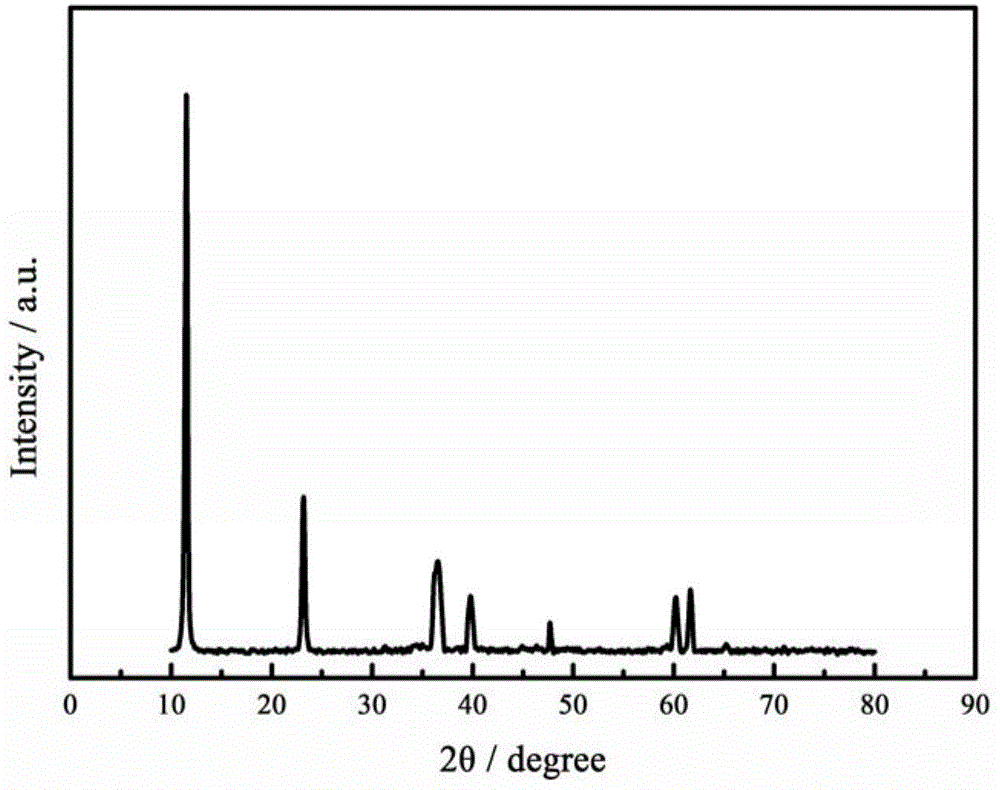
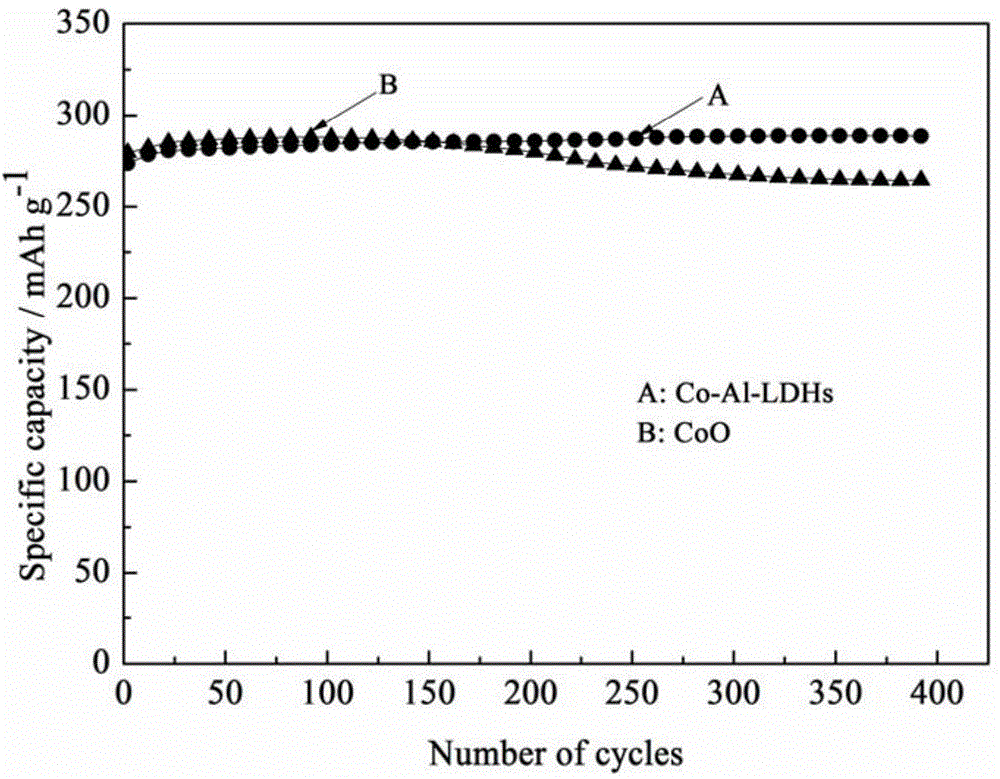
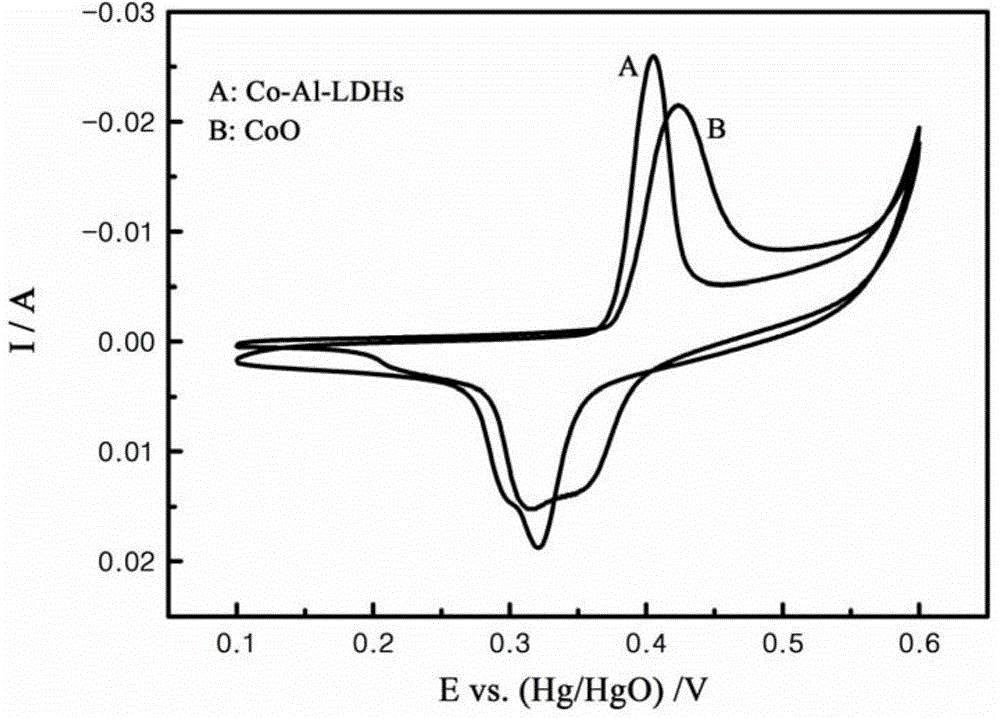
Method for preparing nickel positive electrode of nickel-metal hydride secondary battery by using cobalt-aluminum hydrotalcite and application thereof
A secondary battery, hydrotalcite technology, applied in battery electrodes, nickel storage batteries, alkaline storage battery electrodes and other directions, can solve the problems of complex synthesis, high process conditions, etc., achieve simple synthesis process, controllable material morphology, conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 7.47 parts by weight of analytically pure cobalt nitrate in 100 parts by weight of deionized water; dissolve 3.75 parts by weight of analytically pure aluminum nitrate in 100 parts by weight of deionized water, and mix the above two solutions evenly to form a salt solution . 3.2 parts by weight of sodium hydroxide and 2.12 parts by weight of sodium carbonate were dissolved in 50 parts by weight of deionized water to form a mixed alkali solution.
[0034] Under strong mechanical stirring, slowly add the salt solution and alkali solution dropwise into 50 parts by weight of deionized water, control the pH value of the solution to 9-10, and the temperature to 60-80°C, and continue to stir for 90 minutes. Co-precipitation method React for 4-6 hours, age for 15-20 hours; finally filter the precipitate, wash with distilled water until the filtrate is neutral, and dry at 50-100°C for 8-10 hours to obtain the molar ratio of cobalt ions to aluminum ions It is a 4:1 coba...
Embodiment 2
[0037] Dissolve 9.96 parts by weight of analytically pure cobalt nitrate in 100 parts by weight of deionized water; dissolve 3.75 parts by weight of analytically pure aluminum nitrate in 100 parts by weight of deionized water, and mix the above two solutions evenly to form a salt solution . 3.2 parts by weight of sodium hydroxide and 2.12 parts by weight of sodium carbonate were dissolved in 50 parts by weight of deionized water to form an alkaline solution.
[0038] Under strong mechanical stirring, slowly drop the salt solution and alkali solution into 50 parts by weight of deionized water, control the pH of the solution to 9-10, continue to stir for 90 minutes, age for 18 hours, and finally filter and wash the precipitate , and dried at 80°C for 4 hours to obtain a cobalt-aluminum hydrotalcite sample with a molar ratio of cobalt ions to aluminum ions of 4:1.
[0039] 0.4g cobalt aluminum hydrotalcite, 0.5g nickel hydroxide, 0.04g graphite, 0.04g acetylene black, 0.01g sodi...
Embodiment 3
[0041] Dissolve 12.45 parts by weight of analytically pure cobalt nitrate in 100 parts by weight of deionized water; dissolve 3.75 parts by weight of analytically pure aluminum nitrate in 100 parts by weight of deionized water, and mix the above two solutions evenly to form a salt solution . 3.2 parts by weight of sodium hydroxide and 2.12 parts by weight of sodium carbonate were dissolved in 50 parts by weight of deionized water to form an alkaline solution.
[0042] Under strong mechanical stirring, slowly drop the salt solution and alkali solution into 50 parts by weight of deionized water, control the pH of the solution to 9-10, continue to stir for 90 minutes, age for 18 hours, and finally filter and wash the precipitate , and dried at 80°C for 4 hours to obtain a cobalt-aluminum hydrotalcite sample with a molar ratio of cobalt ions to aluminum ions of 5:1.
[0043]Put 0.35g of cobalt aluminum hydrotalcite, 0.55g of nickel hydroxide, 0.05g of acetylene black, 0.025g of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com