Method for treating potassium chloride residues in organic fluorination reaction
A treatment method, potassium chloride technology, applied in the chemical field, can solve problems such as limited application fields and increased fluorination costs, achieve considerable economic benefits, and eliminate the risk of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
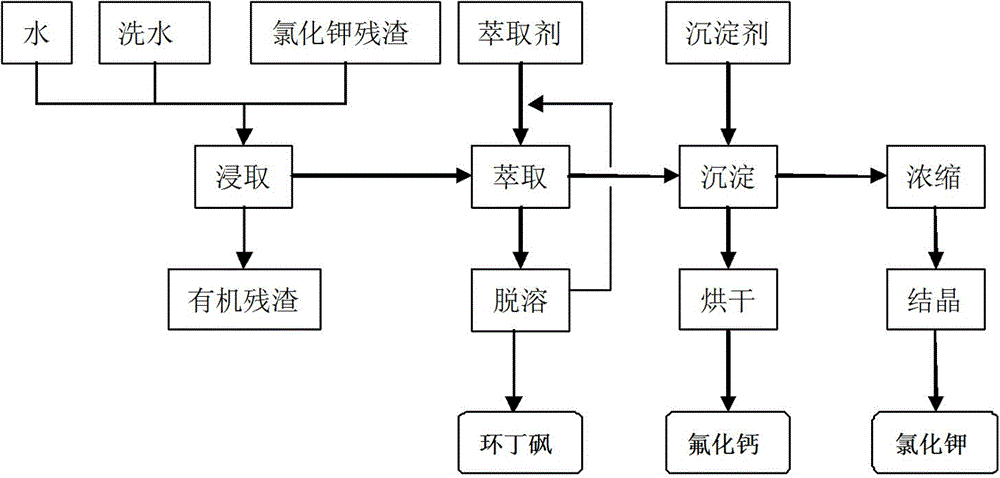
[0012] Example 1: In a 1000ml beaker, add 600ml water and 300g potassium chloride residue, stir and leach for 50min at room temperature, suction filter, transfer the leaching solution into a 1000ml beaker, wash the filter residue twice with 50ml hot water, and dry to obtain a dry filter residue 21g, washed with water for next leaching; the leaching solution was extracted twice with 500ml toluene, and after the oil phase was detoluene, 9g of sulfolane was obtained, and the toluene was recycled; 126ml of calcium chloride solution with a mass concentration of 30% was added to the water phase at room temperature , stirred to complete the precipitation reaction, separated the precipitate, washed with hot water, and dried to obtain 24g of white powdery calcium fluoride, with a content of 97%; evaporated and concentrated the separated liquid to nearly dryness, filtered, and dried to obtain 224g of white crystal potassium chloride, with a content of 98%.
Embodiment 2
[0013] Example 2: In a 1000ml beaker, add 400ml of water and 300g of potassium chloride residue, heat up to 50°C, stir and leach for 10 minutes, filter with suction, transfer the leaching solution into a 1000ml beaker, wash the filter residue twice with 50ml of hot water, and dry Obtain 23g of dry filter residue, and wash with water for the next leaching; the leaching solution is extracted twice with 200ml chloroform, and after the oil phase is removed from chloroform, 8g of sulfolane is obtained, and the chloroform is recycled; ℃, add 15g of calcium oxide powder, stir to complete the precipitation conversion reaction, separate the precipitate, wash with hot water, and dry to obtain 22g of white powdery calcium fluoride, with a content of 96%; evaporate and concentrate the separated liquid to nearly dryness, filter and dry to obtain White crystalline potassium chloride 221g, content 98.5%.
Embodiment 3
[0014] Example 3: In a 1000ml beaker, add 600ml of water and 300g of potassium chloride residue, heat up to 40°C, stir and leach for 20 minutes, filter with suction, transfer the leaching solution into a 1000ml beaker, wash the filter residue twice with 50ml of hot water, and dry Obtain 21g of dry filter residue, wash with water for the next leaching; the leaching solution is extracted twice with 300ml carbon tetrachloride, and after the oil phase removes carbon tetrachloride, 7g of sulfolane is obtained, and the carbon tetrachloride is recycled; Add 18g of calcium hydroxide at 40°C, stir to complete the precipitation transformation reaction, separate the precipitate, wash with hot water, and dry to obtain 22g of white powdery calcium fluoride, with a content of 96%; evaporate and concentrate the separated liquid to nearly dryness, filter and dry to obtain White crystalline potassium chloride 223g, content 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com