Ru-based core-shell catalyst and its preparation method and use in methane oxidation reforming preparation of synthetic gas
A catalytic oxidation and catalyst technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, silicon compounds, etc., can solve the problems of high initial activity, limited resources, easy deactivation, etc. Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
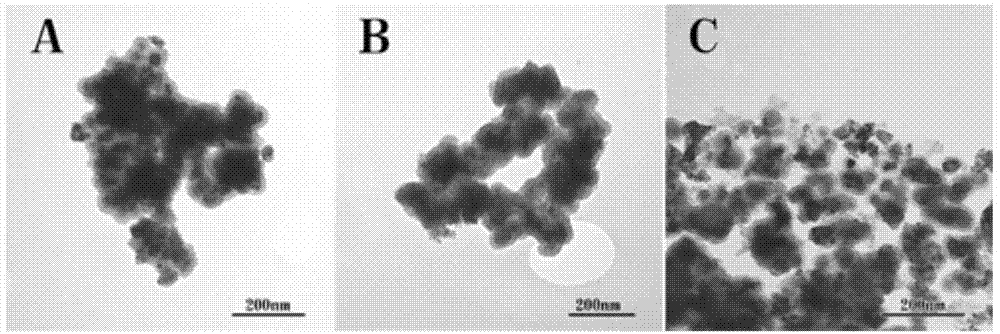
[0036] Add 0.4g of cetyltrimethylammonium bromide (CTAB) to 50ml of distilled water, stir at 50°C for 1h, then add 0.1g of RuCl 3 , stirred for 1 h, added dropwise hydrazine hydrate (diluted 1 ml hydrazine hydrate to 9 ml distilled water, and adjusted pH=10 with 1 mol / L NaOH) into the three-necked flask, and reacted for 1 h. The product was separated by centrifugation with H 2 O and EtOH were washed three times each, dried at 80 °C, and calcined at 500 °C in air for 3 h. Take 0.2g of ruthenium oxide nanoparticles prepared above, add them to 60ml of absolute ethanol, and after ultrasonic treatment (KQ-100DE, 40kHz, 100W) for 0.5h, add 6ml of NH 3 ·H 2 O Continue to sonicate for 0.5h, then add 0.0624g of tetraethyl orthosilicate (TEOS) to make Si / Ru=0.2, continue to sonicate for 1h, wash by centrifugation, and dry at 100°C. The dried catalyst was pressed into tablets and crushed to obtain 40-60 mesh particles for activity testing.
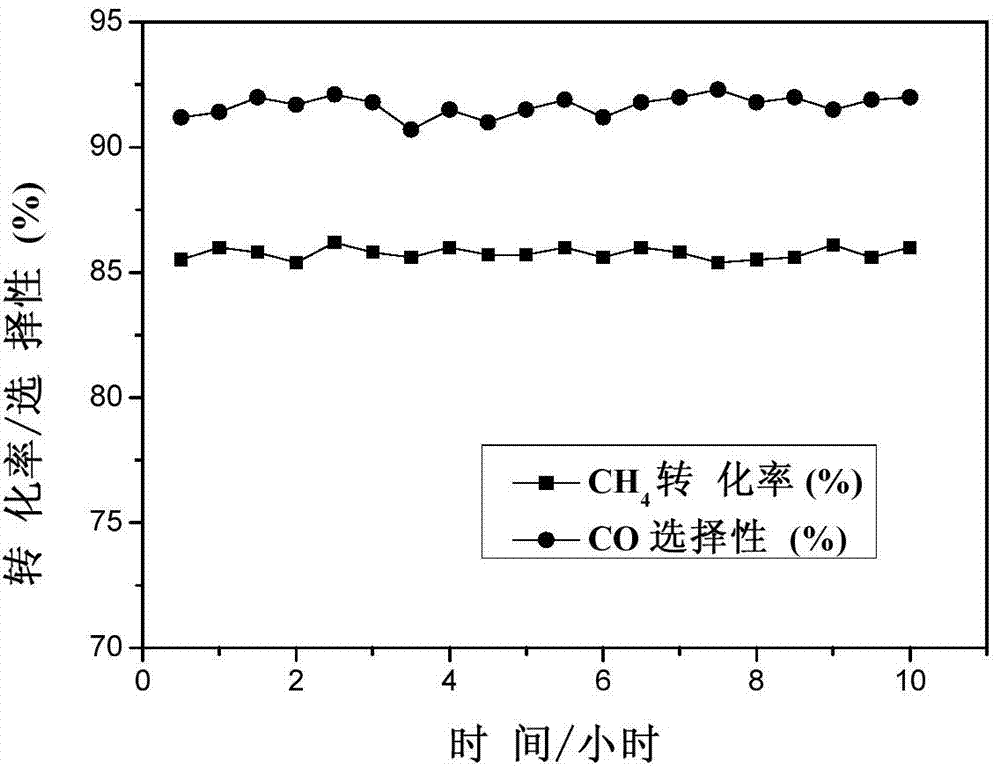
[0037] Take 50 mg of the above-mentioned c...
Embodiment 2
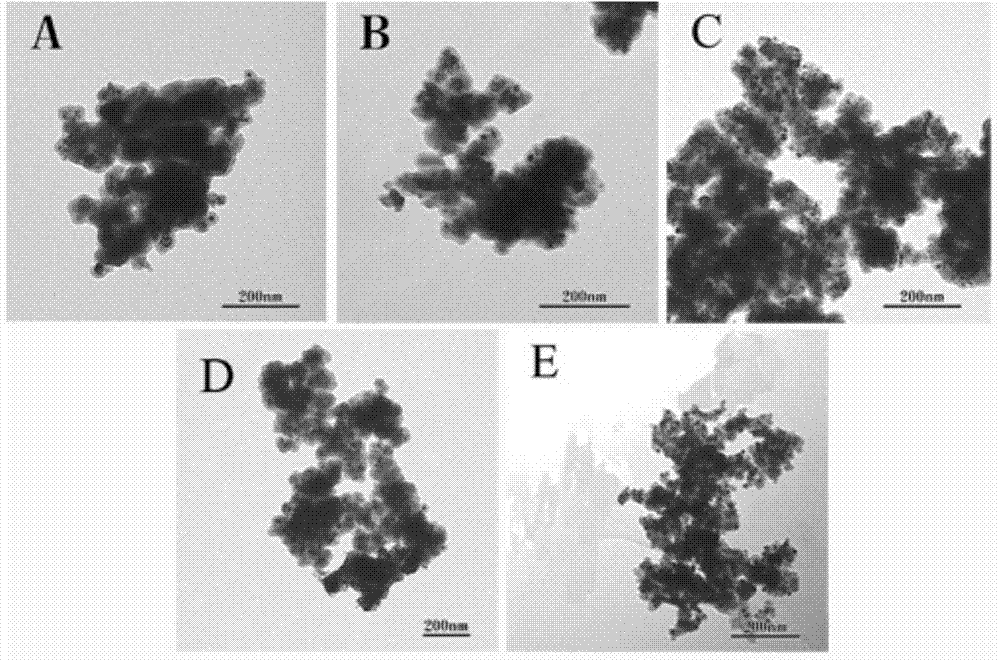
[0039] Add 0.4g CTAB to 50ml distilled water, stir at 50°C for 1h, then add 0.1g RuCl 3 , stirred for 1 h, added dropwise hydrazine hydrate (diluted 1 ml hydrazine hydrate to 9 ml distilled water, and adjusted pH=10 with 1 mol / L NaOH) into the three-necked flask, and reacted for 1 h. The product was separated by centrifugation with H 2 O and EtOH were washed three times each, dried at 80 °C, and calcined at 500 °C in air for 3 h. Take 0.2g of ruthenium oxide nanoparticles prepared above, add them to 60ml of absolute ethanol, and after ultrasonic treatment (KQ-100DE, 40kHz, 100W) for 0.5h, add 6ml of NH 3 ·H 2 O Continue to sonicate for 0.5h, then add 0.0315g TEOS to make Si / Ru=0.1, continue to sonicate for 1h, wash by centrifugation, and dry at 100°C. The dried catalyst was pressed into tablets and crushed to obtain 40-60 mesh particles for activity testing.
[0040] Take 50 mg of the above-mentioned catalyst, and carry out the continuous reaction of methane partial oxidat...
Embodiment 3
[0042] Add 0.4g CTAB to 50ml distilled water, stir at 50°C for 1h, then add 0.1g RuCl 3 , stirred for 1 h, added dropwise hydrazine hydrate (diluted 1 ml hydrazine hydrate to 9 ml distilled water, and adjusted pH=10 with 1 mol / L NaOH) into the three-necked flask, and reacted for 1 h. The product was separated by centrifugation with H 2 O and EtOH were washed three times each, dried at 80 °C, and calcined at 500 °C in air for 3 h. Take 0.2g of ruthenium oxide nanoparticles prepared above, add them to 60ml of absolute ethanol, and after ultrasonic treatment (KQ-100DE, 40kHz, 100W) for 0.5h, add 6ml of NH 3 ·H 2 O Continue to sonicate for 0.5h, then add 0.0933g TEOS to make Si / Ru=0.3, continue to sonicate for 1h, wash by centrifugation, and dry at 100°C. The dried catalyst was pressed into tablets and crushed to obtain 40-60 mesh particles for activity testing.
[0043] Take 50 mg of the above-mentioned catalyst, and carry out the continuous reaction of methane partial oxidat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com