Preparation method for substituted cinnamyl alcohol
A technology of cinnamyl alcohol and cinnamaldehyde, which is used in pharmaceutical chemical intermediates and related chemical fields, can solve the problems of high price, non-reusable, difficult separation and recovery, etc., and achieves the effects of good reproducibility and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
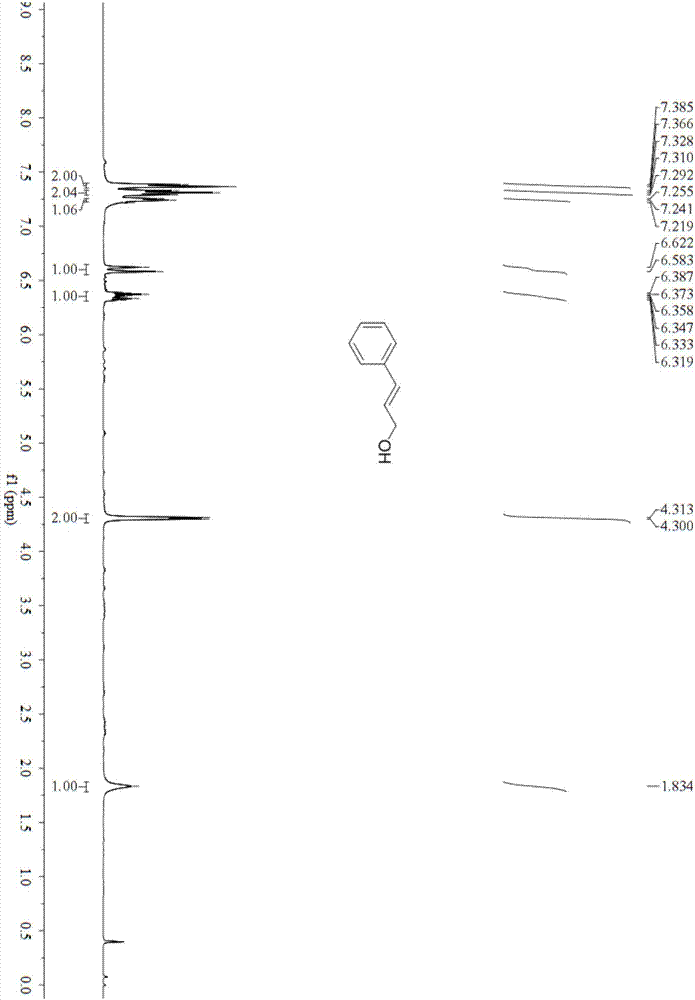
[0023] Embodiment 1: the synthesis of α-methylcinnamyl alcohol
[0024] In the methanol (5mL) solvent that adds AuNPore (10.0mg, 10mol%) catalyst, add substrate α-methylcinnamaldehyde (74.07mg, 0.5mmol), sodium hydroxide (200mg, 5mmol) and triisopropyl Silane (791.8mg, 5.0mmol), placed on a magnetic stirrer at 50°C for 24h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 20:1) to obtain α-formazan Cinnamyl alcohol 45.91mg, yield 62%.
[0025]
[0026] yellow liquid; 1 H NMR (CDCl 3 ,400MHz)δ:7.32(d,J=7.6Hz,2H),7.27(d,J=7.2Hz,2H),7.23–7.20(m,1H),6.52(s,1H),4.17(s,2H ),1.99(br s,1H,OH),1.89(s,3H).
Embodiment 2
[0027] Embodiment 2: the synthesis of α-methylcinnamyl alcohol
[0028] In the methanol (5mL) solvent that adds AuNPore (10.0mg, 10mol%) catalyst, add substrate α-methylcinnamaldehyde (74.07mg, 0.5mmol), potassium hydroxide (280mg, 5mmol) and triisopropyl Silane (791.8mg, 5.0mmol), placed on a magnetic stirrer at 60°C for 24h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 20:1) to obtain α-formazan 48.13 mg of cinnamyl alcohol, yield 65%.
[0029]
[0030] yellow liquid; 1 H NMR (CDCl 3 ,400MHz)δ:7.32(d,J=7.6Hz,2H),7.27(d,J=7.2Hz,2H),7.23–7.20(m,1H),6.52(s,1H),4.17(s,2H ),1.99(br s,1H,OH),1.89(s,3H).
Embodiment 3
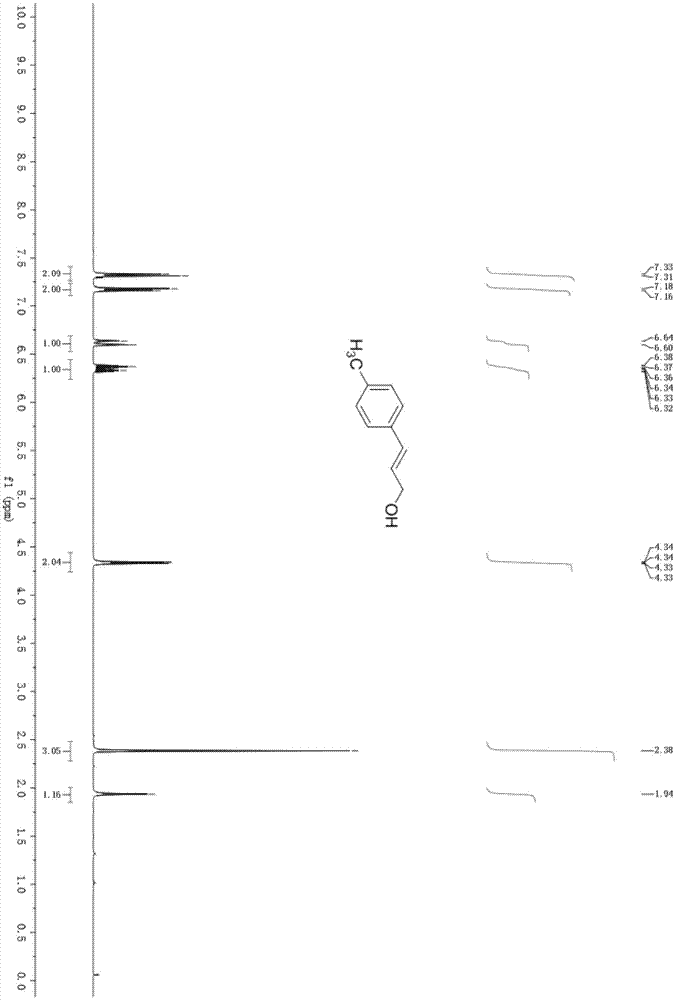
[0031] Embodiment 3: the synthesis of 4-methylcinnamyl alcohol
[0032] In the acetone (5mL) solvent that adds AuNPore (10.0mg, 10mol%) catalyst, add substrate 4-methylcinnamaldehyde (74.07mg, 0.5mmol), sodium hydroxide (200mg, 5mmol) and triisopropyl Silane (791.8mg, 5.0mmol), placed on a magnetic stirrer at 50°C for 24h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether: ethyl acetate = 20:1) to obtain 4-formazan 45.91 mg of cinnamyl alcohol, yield 70%.
[0033]
[0034] colorless solid; 1 H NMR (CDCl 3,400MHz)δ:7.32(d,J=8.0Hz,2H),7.16(d,J=8.0Hz,2H),6.62(d,J=16.0Hz,1H),6.35(dt,J=16.0,4.0 Hz, 1H), 4.33(dd, J=4.0, 2.0Hz, 2H), 2.38(s, 3H), 1.94(br s, 1H, OH); m.p., 50–52℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com