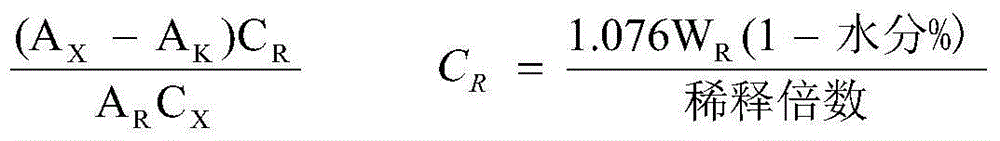Bromfenac sodium eye drops and preparation method thereof
A technology of bromfenac sodium and eye drops, which can be used in pharmaceutical formulations, sensory diseases, non-central analgesics, etc., can solve the problems of short adhesion time, change the viscosity of eye drops, etc., and achieve increased safety and efficiency. Effectiveness, improvement of polymer impurities, effect of prolonging residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
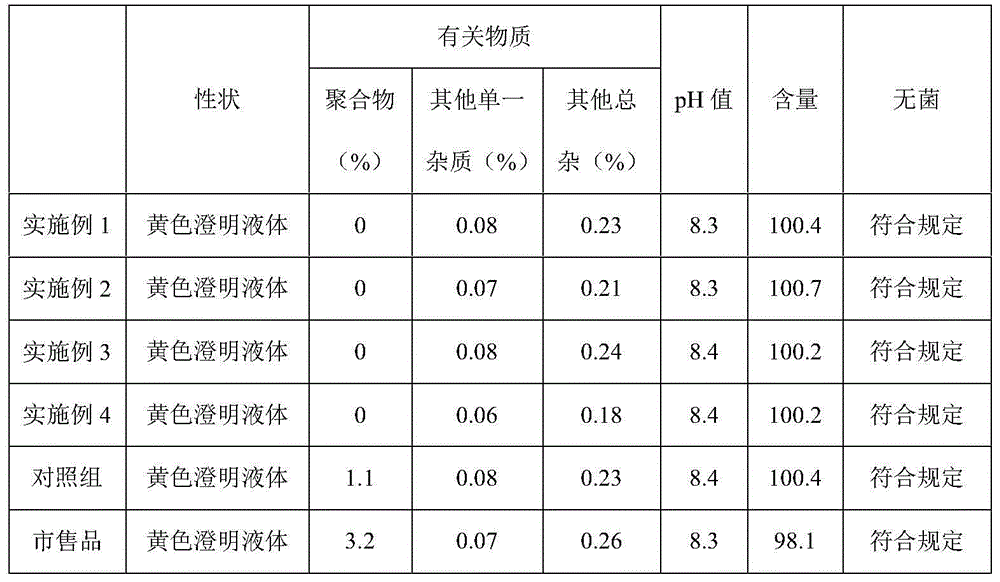
Embodiment 1
[0019] Prescription: bromfenac sodium 5g, molecular weight range 1.5×10 6 About 5g of sodium hyaluronate, 27.9g of boric acid, 52.5g of borax, 0.5g of benzalkonium bromide, 0.5g of anhydrous sodium sulfite, 0.5g of disodium edetate, and 5000ml of water for injection were added to make 1000 sticks.
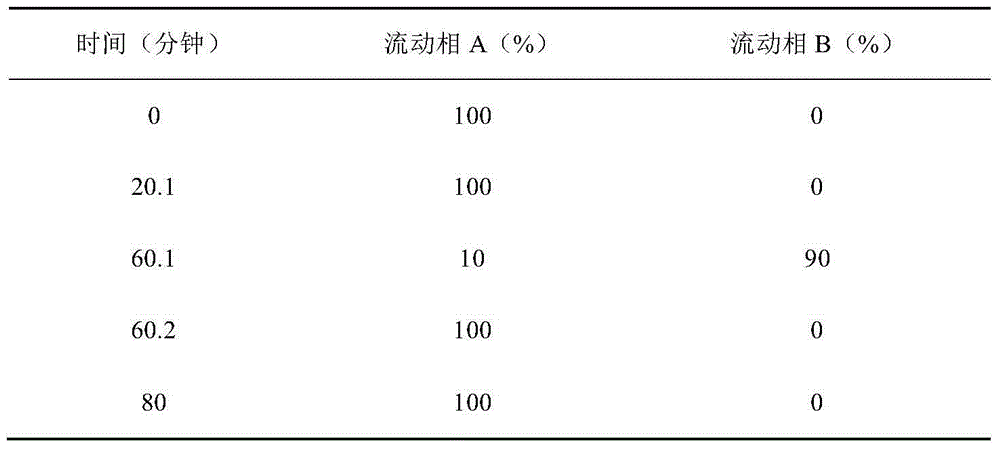
[0020] The preparation method is as follows: 1) disperse the prescribed amount of sodium hyaluronate in an appropriate amount of water for injection at 40°C to 50°C, and make it swell to form a transparent solution; Add disodium sulfite and benzalkonium bromide, and add 1), heat to boil, boil for 30 minutes, cool to 30-40°C, add anhydrous sodium sulfite and bromfenac sodium respectively, stir for 15 minutes to dissolve, add injection Add water to the full volume, mix for 15 minutes, and adjust the pH to 8.3 with sodium hydroxide. Filter cycle for 15 minutes. After taking samples to measure the content and pH, they were filled and sealed in 5ml plastic bottles for eye drops cleane...
Embodiment 2
[0022] Prescription: Bromfenac Sodium 5g, Hydroxypropyl Methylcellulose 1g, Carbomer 1g, Sodium Citrate 27.9g, Disodium Hydrogen Phosphate 52.5g, Methylparaben 0.2g, Methylparaben Propyl Add 0.3g, 0.2g p-hydroxytert-butanol anisole, 0.3g propyl gallate, 0.4g triethanolamine, 0.2g tryptophan, and water for injection to 5000ml, and make 1000 tubes in total.
[0023] The preparation method is as follows: 1) Disperse the prescribed amount of hydroxypropyl methylcellulose and carbomer in an appropriate amount of water for injection at 40°C to 50°C to swell to form a transparent solution; 2) Add the prescription water under stirring Amount of sodium citrate, disodium hydrogen phosphate, triethanolamine, tryptophan, paraben, methyl paraben propyl, add 1), heat to boiling, after boiling for 30 minutes, cool to 30-40°C , add p-hydroxytert-butanol anisole, propyl gallate, and bromfenac sodium respectively, stir for 15 minutes to dissolve, add water for injection to the full amount, mix ...
Embodiment 3
[0025] Prescription: bromfenac sodium 5g, molecular weight range 1.2×10 6 About 8g of sodium hyaluronate, 27.9g of boric acid, 52.5g of borax, 0.5g of benzalkonium bromide, 0.5g of anhydrous sodium sulfite, 0.5g of disodium edetate, and water for injection were added to 5000ml, and a total of 1000 tubes were prepared.
[0026] The preparation method is as follows: 1) disperse the prescribed amount of sodium hyaluronate in an appropriate amount of water for injection at 40°C to 50°C, and make it swell to form a transparent solution; Add disodium sulfite and benzalkonium bromide, and add 1), heat to boil, boil for 30 minutes, cool to 30-40°C, add anhydrous sodium sulfite and bromfenac sodium respectively, stir for 15 minutes to dissolve, add injection Add water to the full volume, mix for 15 minutes, and adjust the pH to 8.3 with sodium hydroxide. Filter cycle for 15 minutes. After taking samples to measure the content and pH, they were filled and sealed in 5ml plastic bottles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com