Synthesis method for di-tert-butyl terminated chain polythiaether
A technology of di-tert-butyl sealing and synthesizing method is applied in the field of preparation of organic sulfides, and can solve the problems of low isobutene conversion rate and product yield, low alumina activity, and difficult recycling of catalysts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
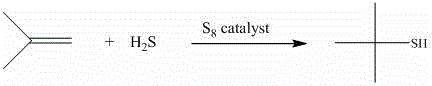
[0030] Add 367.0 g (11.44mol) sulfur into a 2.5-liter reactor, and add 0.734 g NH 2 -MIL-53 (Al) catalyst (0.2% by mass based on sulfur), then reinforced and sealed. Purge 3 times with 2.0 MPa high purity nitrogen, and finally release to normal pressure. Pass H into the above reaction kettle 2 S (129.8 g, 3.81 mol), heat up and control the temperature to 140 oC, and start stirring (about 320rpm). Start the metering pump and inject isobutene (427.6 g, 7.62mol). Adjust the flow rate of the metering pump so that the injection time of isobutene is 1h. After the injection of isobutene, the reaction will continue for 1h. At this time, the pressure of the reactor drops to 0.3MPa, and the temperature is lowered to 100℃. Constant at this temperature, release the pressure in the kettle and purge it with nitrogen for 0.5h. The tail gas is cooled with a primary condenser (ice water bath) and a secondary condenser (dry ice acetone bath) to recover by-product tert-butyl mercaptan and di-tert...
Embodiment 2
[0032] Add 555.84g (17.37 mol) of sulfur into a 2.5-liter reactor, and add 0.556 g of NH 2 -MIL-101 (Al) Catalysis
[0033]
[0034] When the molar ratio of hydrogen sulfide, sulfur and isobutylene is 1:3:2, the average chain length of the product is 4, and the product is mainly tetrasulfide. When the molar ratio of hydrogen sulfide, sulfur and isobutylene is 1:2: At 2 o'clock, the average chain length of the product is 3, and the product is mainly trisulfide.
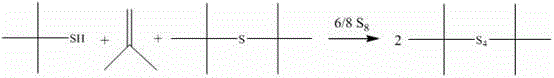
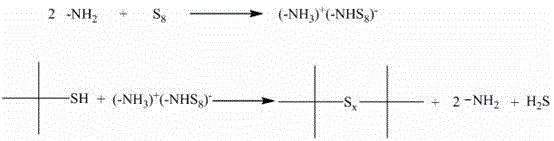
[0035] Although isobutylene can be converted 100%, the by-products tert-butyl mercaptan and di-tert-butyl monosulfide are inevitably formed. Nitrogen stripping is carried out at 100°C, and the exhaust gas is passed through a primary condenser (ice water bath) and secondary The condenser (dry ice acetone bath) cools and recovers the by-products tert-butyl mercaptan and di-tert-butyl monosulfide, which can continue to react with sulfur to produce chain di-tert-butyl polysulfide, the reaction formula is as follows:
[0036]
[...
Embodiment 3
[0039] Add 243.8 g (7.62 mol) of sulfur into a 2.5-liter reactor and add 0.975 g of NH 2 -MIL-101 (Al) catalyst (0.4% based on the mass percentage of sulfur), and then reinforced and sealed. Purge 3 times with 2.0 MPa high purity nitrogen, and finally release to normal pressure. Pass H into the above reaction kettle 2 S (129.8 g, 3.81 mol), heat up and control the temperature to 150 oC, start stirring (about 320rpm). Start the metering pump and inject isobutene (427.6 g, 7.62mol). Adjust the flow rate of the metering pump so that the injection time of isobutene is 1h. After the injection of isobutene, the reaction will continue for 1h. At this time, the pressure of the reactor drops to 0.3MPa, and the temperature is lowered to 100℃. Constant at this temperature, release the pressure in the kettle and purge it with nitrogen for 0.5h. The tail gas is cooled with a primary condenser (ice water bath) and a secondary condenser (dry ice acetone bath) to recover by-product tert-butyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com