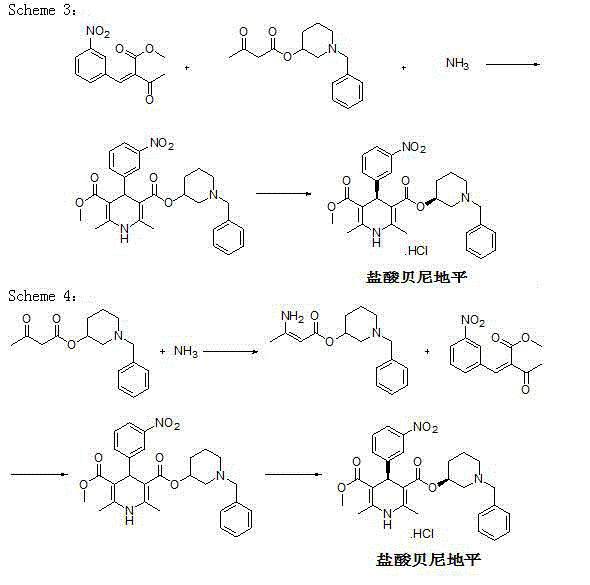
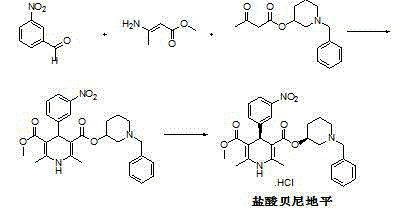
Synthetic method for benidipine hydrochloride intermediate
A technology of a dipine intermediate and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of waste of raw materials, few reports on the preparation method of compound A, increase the difficulty of purification, etc., and achieve the effects of high yield and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
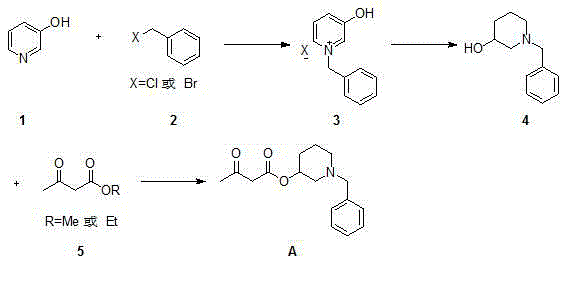
[0030] Preparation of 1-benzyl-3-hydroxypyridine halide:
[0031] Add methanol (10 L), 3-hydroxypyridine (1 kg) and benzyl bromide (2.15 kg) into a stirred 20 L reaction flask in sequence, heat to reflux, and complete the reaction in 3 hours, then directly concentrate under reduced pressure and distill out the methanol , washed with petroleum ether, filtered, and dried to obtain 2.73 kg of white solid.
[0032] LCMS (ESI): 186 [M-Br - ]
[0033] Add acetonitrile (150 mL), 3-hydroxypyridine (10 g) and benzyl chloride (19.9 g) to a 250 mL reaction flask in sequence, heat to reflux, and the reaction is complete in 2 hours, and directly concentrate under reduced pressure and distill out acetonitrile. Slurry and wash with petroleum ether, filter, and dry to obtain 22.6 g of white solid.
Embodiment 2
[0035] Preparation of 1-benzyl-3-piperidinol:
[0036] Add methanol (10 L) and 1-benzyl-3-hydroxypyridinium bromide (500 g) into the reaction flask, add sodium borohydride (215 g) in batches, the temperature does not exceed 25 ° C, after the addition, Heat to reflux, react for 4 hours, the reaction is complete, cool to 0~10°C, add hydrochloric acid dropwise, adjust the pH value to 8, concentrate under reduced pressure to remove the solvent, add ethyl acetate and saturated saline to dissolve, separate the liquid, and use ethyl acetate The product in the aqueous phase was extracted twice with ester, and the ethyl acetate phase was combined, dried over anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain 342.6 g of light yellow oil.
[0037] LCMS (ESI): 192 [M+H + ]
[0038] Add ethanol (1 L) and 1-benzyl-3-hydroxypyridinium chloride (50 g) to the reaction flask, add sodium borohydride (43 g) in batches, the temperature does not exceed 25 ° C, after the add...
Embodiment 3
[0040] Preparation of (1-benzyl-3-piperidinyl)acetoacetate:
[0041] Add 1-benzyl-3-piperidinol (600 g), toluene (6 L), ethyl acetoacetate (490.2 g), and boric acid (19.5 g) into a 10 L reaction flask, heat to reflux overnight, and perform HPLC Detection, 1-benzyl-3-piperidinol remaining 26.2%, add atmospheric distillation device, steam the ethanol generated by the reaction, complete the reaction in 2 hours, cool to 0~10°C, adjust the pH value to about 3, Extract the product with water (1 L×2), combine the aqueous phases, and adjust the pH value of the aqueous phase to about 7, extract with ethyl acetate (1 L×2), combine the organic phases, and wash with saturated brine (500 mL× 2) Wash, dry with anhydrous sodium sulfate, filter, and concentrate to dryness to obtain 786.1 g of yellow oil.
[0042] LCMS (ESI): 276 [M+H + ]
[0043] 1 HNMR (DMSO, 400M): δ=1.26-1.83(m,4H), 2.09-2.13(m,2H), 2.17(s,3H), 2.50-2.53(m,1H), 2.66-2.80(m, 1H), 3.56(s, 2H), 3.48(s, 2H), 4.72-4.76(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com