Method for preparing arene by directly converting methane
A technology for methane and aromatic hydrocarbons, applied in the field of direct conversion of methane to aromatic hydrocarbons, can solve the problems of low catalyst activity, limited effect, catalyst deactivation, etc., and achieves the advantages of inhibiting the formation of oligomeric condensed aromatic hydrocarbons, prolonging the service life and improving the conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
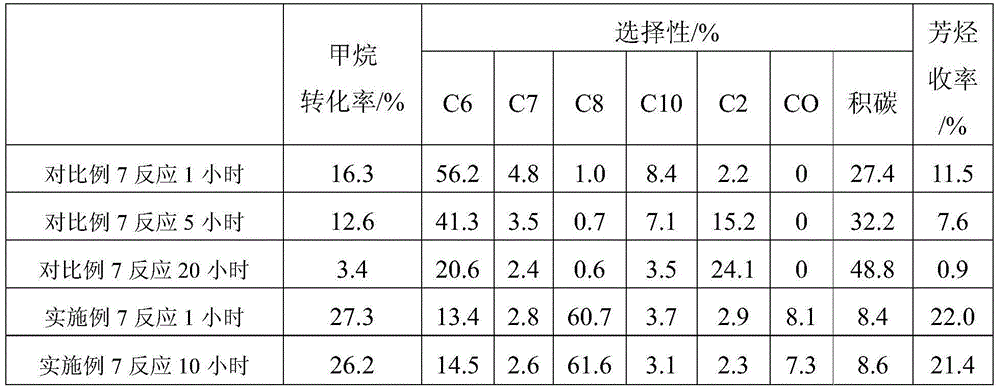
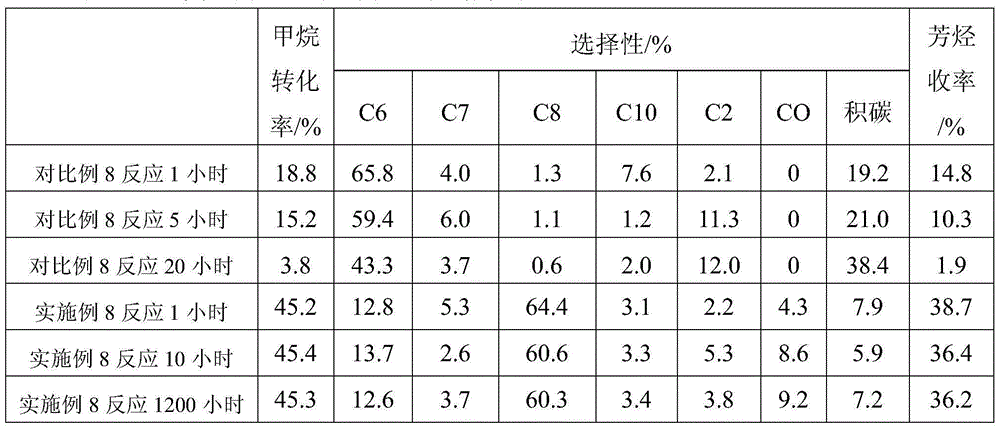
Examples
Embodiment 1
[0028] The carrier of catalyst of the present invention can adopt existing commercial goods, also can directly prepare by following method:
[0029] Take 16.288g template agent tetrapropylammonium hydroxide (TPAOH), add 42.084g water, stir on a magnetic stirrer at room temperature, then add 1.8757g aluminum nitrate, stir until completely dissolved, then add 20.833g orthosilicate dropwise Ethyl ester (TEOS) with constant stirring, after being fully hydrolyzed, stirred for 20 hours, left to stand and aged for 4 hours, the resulting synthetic solution was slowly poured into a stainless steel autoclave lined with polytetrafluoroethylene, and sealed. Then put the stainless steel autoclave in an oven at 120°C for crystallization for 120 hours, take it out, and cool it down to room temperature. The obtained product was repeatedly washed with deionized water until the pH value was 9-10, and the solid product was separated by centrifugation. The obtained product was dried in an oven at...
Embodiment 2
[0031] The carrier of catalyst of the present invention can adopt existing commercial goods, also can directly prepare by following method:
[0032]Take 16.288g template agent tetrapropylammonium hydroxide (TPAOH), add 42.084g water, stir on a magnetic stirrer at room temperature, then add 0.9378g aluminum nitrate, stir until completely dissolved, then add 20.833g orthosilicate dropwise Ethyl ester (TEOS) with constant stirring, after being fully hydrolyzed, stirred for 20 hours, left to stand and aged for 4 hours, the resulting synthetic solution was slowly poured into a stainless steel autoclave lined with polytetrafluoroethylene, and sealed. Then put the stainless steel autoclave in an oven at 120°C for crystallization for 120 hours, take it out, and cool it down to room temperature. The obtained product was repeatedly washed with deionized water until the pH value was 9-10, and the solid product was separated by centrifugation. The obtained product was dried in an oven at ...
Embodiment 3
[0034] The carrier of catalyst of the present invention can adopt existing commercial goods, also can directly prepare by following method:
[0035] Take 16.288g template agent tetrapropylammonium hydroxide (TPAOH), add 42.084g water, stir on a magnetic stirrer at room temperature, then add 0.7504g aluminum nitrate, stir until completely dissolved, then add 20.833g orthosilicate dropwise Ethyl ester (TEOS) with constant stirring, after being fully hydrolyzed, stirred for 20 hours, left to stand and aged for 4 hours, the resulting synthetic solution was slowly poured into a stainless steel autoclave lined with polytetrafluoroethylene, and sealed. Then put the stainless steel autoclave in an oven at 120°C for crystallization for 120 hours, take it out, and cool it down to room temperature. The obtained product was repeatedly washed with deionized water until the pH value was 9-10, and the solid product was separated by centrifugation. The obtained product was dried in an oven at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com