Novel esomeprazole compound entity as well as preparation method and combined pharmaceutical preparation thereof
A new technology for esomeprazole and omeprazole, applied in the field of medicine, can solve the problems of restricting the use of esomeprazole, prone to toxic side effects, low utilization rate and the like, and achieves good shape and is not easy to degrade. , the effect of less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
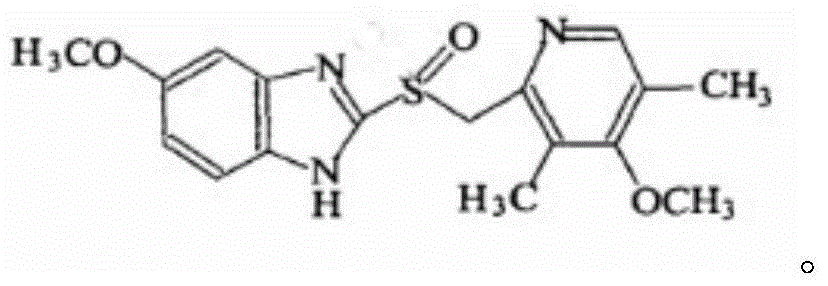
[0030] A new compound entity of esomeprazole, its chemical structural formula is
[0031]
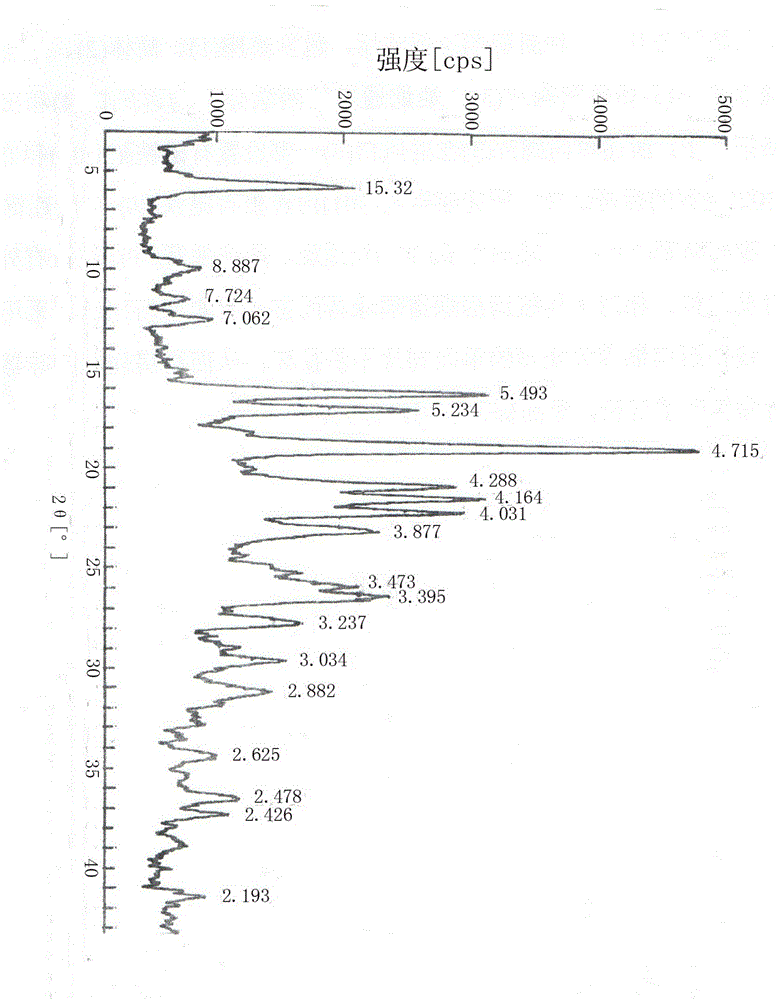
[0032]The new esomeprazole compound entity is determined by X-ray powder diffraction, and its spectrum has the following diffraction angle 2θ, with an error of ±0.2°, expressed in angles as 5.8°, 10.0°, 11.5°, 12.6°, 16.2°, 17.0°, 18.9°, 20.9°, 21.5°, 22.2°, 23.1°, 25.8°, 26.4°; the corresponding D values are 15.32, 8.887, 7.724, 7.062, 5.493, 5.234, 4.715,
[0033] 4.288, 4.164, 4.031, 3.877, 3.473, 3.395.
[0034] The preparation method of above-mentioned esomeprazole novel compound entity, the method comprises the steps:
[0035] Add 360 grams of 5-methoxy-2-mercapto-1H-benzimidazole and 444 grams of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride in the reactor, and then Add 3600 grams (4547ml) of anhydrous methanol, and heat to reflux to clarify the solution. Then add 1257 grams of 14% sodium hydroxide solution, heat and reflux for 4 hours, after the reaction is...
Embodiment 2
[0042] A new compound entity of esomeprazole, its chemical structural formula and X-diffraction powder diffraction pattern are as in Example 1.
[0043] The preparation method of above-mentioned esomeprazole novel compound entity, the method comprises the steps:
[0044] Add 360 grams of 5-methoxy-2-mercapto-1H-benzimidazole and 444 grams of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride in the reactor, and then Add 3780 grams (4774ml) of anhydrous methanol and heat to reflux to clarify the solution. Then add 1227 grams of 15% sodium hydroxide solution, heat and reflux for 3.8 hours, after the reaction is complete, adjust the pH value to 6-7 with concentrated hydrochloric acid, filter, concentrate under reduced pressure to completely recover the solvent methanol, and use 25.5 kg of dichloromethane (19.2L) was dissolved by heating under reflux with stirring, washed three times with 4500ml of saturated brine, dried with anhydrous sodium sulfate for 2 hours, filtere...
Embodiment 3
[0051] A new compound entity of esomeprazole, its chemical structural formula and X-diffraction powder diffraction pattern are as in Example 1.
[0052] The preparation method of above-mentioned esomeprazole novel compound entity, the method comprises the steps:
[0053] Add 360 grams of 5-methoxy-2-mercapto-1H-benzimidazole and 444 grams of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride in the reactor, and then Add 3960 grams (5000 ml) of anhydrous methanol, and heat to reflux to clarify the solution. Then add 1200 grams of 16% sodium hydroxide solution, heat and reflux for 3.5 hours, after the reaction is complete, adjust the pH value to 6-7 with concentrated hydrochloric acid, filter, concentrate under reduced pressure to completely recover the solvent methanol, and use 26.6 kg of dichloromethane (20L) heated to reflux and stirred to dissolve, washed three times with 5000ml of saturated brine, dried with anhydrous sodium sulfate for 2 hours, filtered, concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com