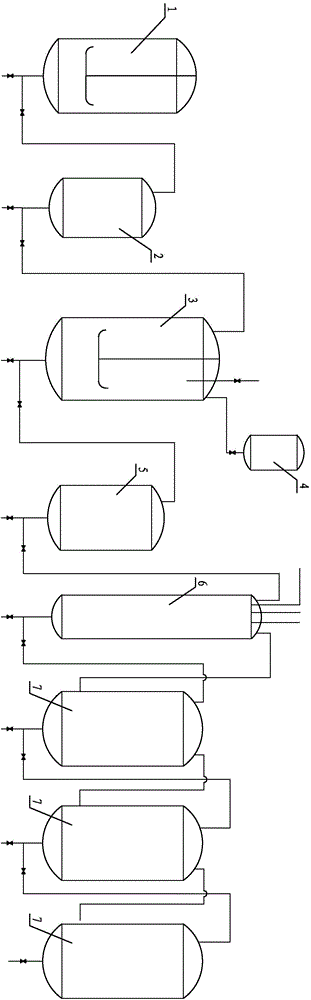
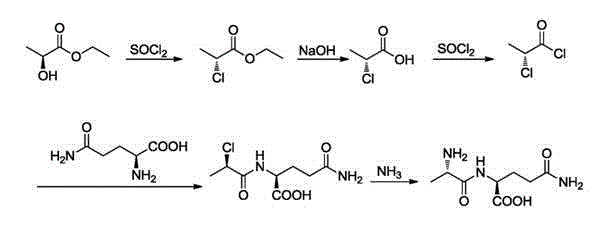
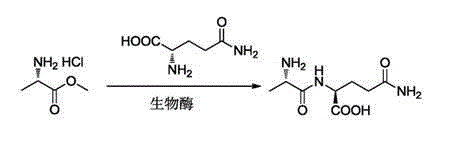
Preparation method and technological system for enzymatically synthesizing N(2)-L-alanyl-L-glutamine
A glutamate dipeptide and process system technology are applied in the preparation field of enzyme catalyzed synthesis of glutamate dipeptide, can solve the problems of affecting the purity and quality of glutamate dipeptide, long reaction synthesis route, large environmental pollution, etc., and achieve high economic value and market competitiveness, low cost, Equipment requires simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]Add 180kg of methanol into the 1000L reactor and cool down to 0°C; add 78.6kg of thionyl chloride dropwise into the reactor at 0°C; add 52.5kg of L-alanine in one go Put it into the reaction kettle, keep it warm and stir for 30min, then slowly raise the temperature to 25°C, stir for 1h, then raise the temperature to 45°C and stir for 2h; evaporate most of the solvent at 50°C under reduced pressure to obtain 90kg of L-alanine methyl ester salt;
Embodiment 2
[0060] Add 180kg of methanol into a 1000L reactor and cool down to 10°C; add 78.6kg of thionyl chloride dropwise into the reactor at 10°C; add lysine to the reactor at one time after the addition of thionyl chloride is complete , keep stirring for 30 minutes, then slowly raise the temperature to 30°C, stir for 1h, then raise the temperature to 50°C and stir for 2h; evaporate most of the solvent at 50°C under reduced pressure to obtain 82L-alanine methyl ester salt;
Embodiment 3
[0062] Add 180kg of methanol into the 1000L reactor, and lower the temperature to 5°C; add 78.6kg of thionyl chloride dropwise into the reactor at 5°C; , keep stirring for 30 minutes, then slowly raise the temperature to 28°C, stir for 1h, then raise the temperature to 48°C and stir for 2h; evaporate most of the solvent at 50°C under reduced pressure to obtain 88kg of L-alanine methyl ester salt;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com