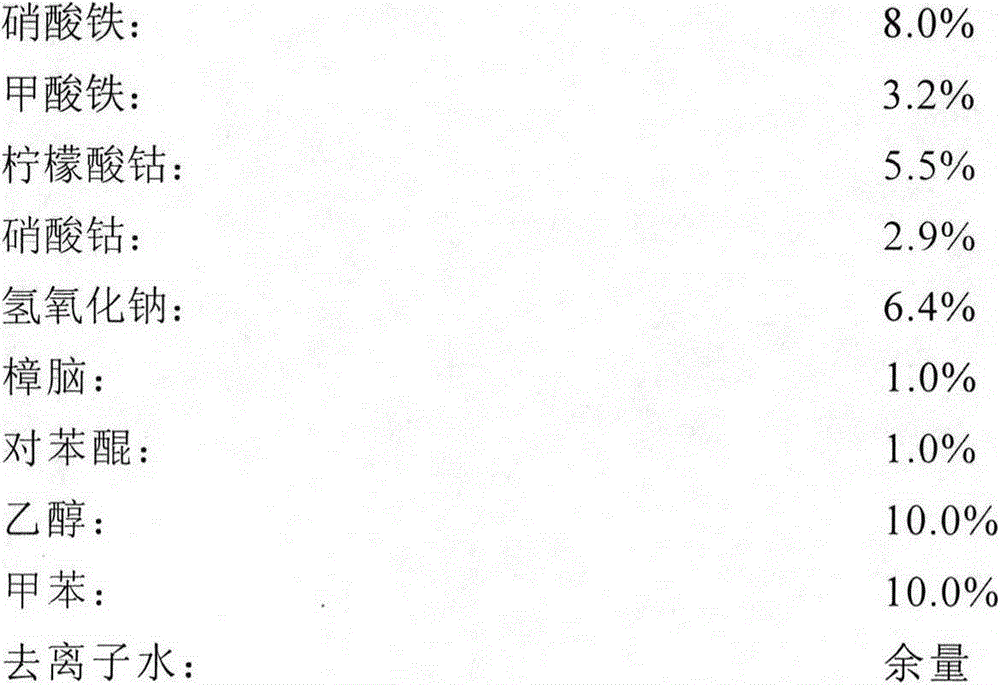Preparation method of porous nano CoFe2O4
A cofe2o4, nanotechnology, applied in the field of photocatalytic materials, to achieve the effects of no surface defects, improved photocatalytic efficiency, and no template residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Porous nano-CoFe 2 o 4 Preparation of:
[0021]
[0022] According to the above mass percentage, ferric trichloride, ferric bromide, cobalt sulfate, cobalt bromide and half of the total amount of deionized water are mixed, stirred and dissolved to obtain ferric chloride, ferric bromide, cobalt sulfate and cobalt bromide Mix the solution, mix potassium hydroxide with the other half of the total amount of deionized water, stir and dissolve to obtain a potassium hydroxide solution, add the mixed solution of ferric chloride, ferric bromide, cobalt sulfate and cobalt bromide dropwise to hydrogen In the potassium oxide solution, continue to stir for 2 hours after the dropwise addition, and the product is subjected to centrifugation and washing operations and repeated three times, and then centrifuged to obtain CoFe 2 o 4 Sol; by CoFe 2 o 4 After mixing the sol and deionized water at a mass ratio of 1:3, disperse for 1 hour with an ultrasonic wave with a frequency of 2...
Embodiment 2
[0024] Porous nano-CoFe 2 o 4 Preparation of:
[0025]
[0026] According to the above mass percentage, mix ferric nitrate, ferric formate, cobalt citrate and cobalt nitrate with half of the total amount of deionized water, stir and dissolve to obtain a mixed solution of ferric nitrate, ferric formate, cobalt citrate and cobalt nitrate. Sodium is mixed with the other half of the total amount of deionized water, stirred and dissolved to obtain a sodium hydroxide solution, and the mixed solution of ferric nitrate, ferric formate, cobalt citrate and cobalt nitrate is added dropwise to the sodium hydroxide solution under stirring. Continue to stir the reaction for 3h, the product is centrifuged and washed and repeated three times, and then centrifuged to obtain CoFe 2 o 4 Sol; by CoFe 2 o 4 After mixing the sol and deionized water at a mass ratio of 1:4, disperse for 2 hours with an ultrasonic wave with a frequency of 68KHz and a power of 3KW, add ethanol, toluene, p-benzo...
Embodiment 3
[0028] Porous nano-CoFe 2 o 4 Preparation of:
[0029]
[0030]
[0031] According to the above mass percentage, iron sulfate, iron citrate, cobalt chloride and cobalt acetate are mixed with half of the total amount of deionized water, stirred and dissolved to obtain a mixed solution of iron sulfate, iron citrate, cobalt chloride and cobalt acetate, and Lithium hydroxide and barium hydroxide are mixed with the other half of the total amount of deionized water, stirred and dissolved to obtain a mixed solution of lithium hydroxide and barium hydroxide, and the mixed solution of iron sulfate, iron citrate, cobalt chloride and cobalt acetate Add dropwise into the mixed solution of lithium hydroxide and barium hydroxide, continue to stir and react for 4 hours after the dropwise addition, the product is subjected to centrifugation and washing operations and repeated three times, and then centrifuged to obtain CoFe 2 o 4 Sol; by CoFe 2 o 4 After mixing the sol and deionize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com