Flavonoid compound, preparation method and application thereof
A compound and composition technology, applied in the field of medicinal chemistry, can solve the problems of carrier materials such as limited drug loading capacity, adverse effects on drug safety and stability, failure to improve the water solubility of dehydrated icariin, etc., to achieve improved Bioavailability, broadening the dosage form of clinical application, and improving the effect of water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
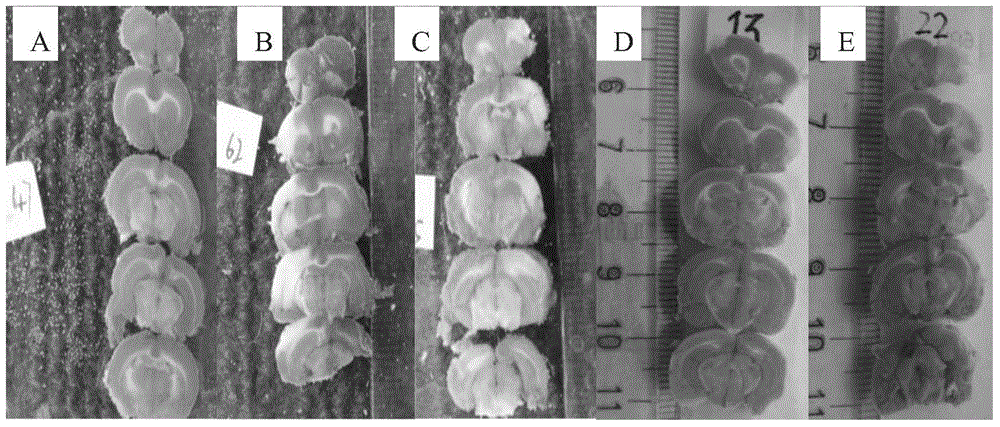
Image
Examples
preparation example Construction
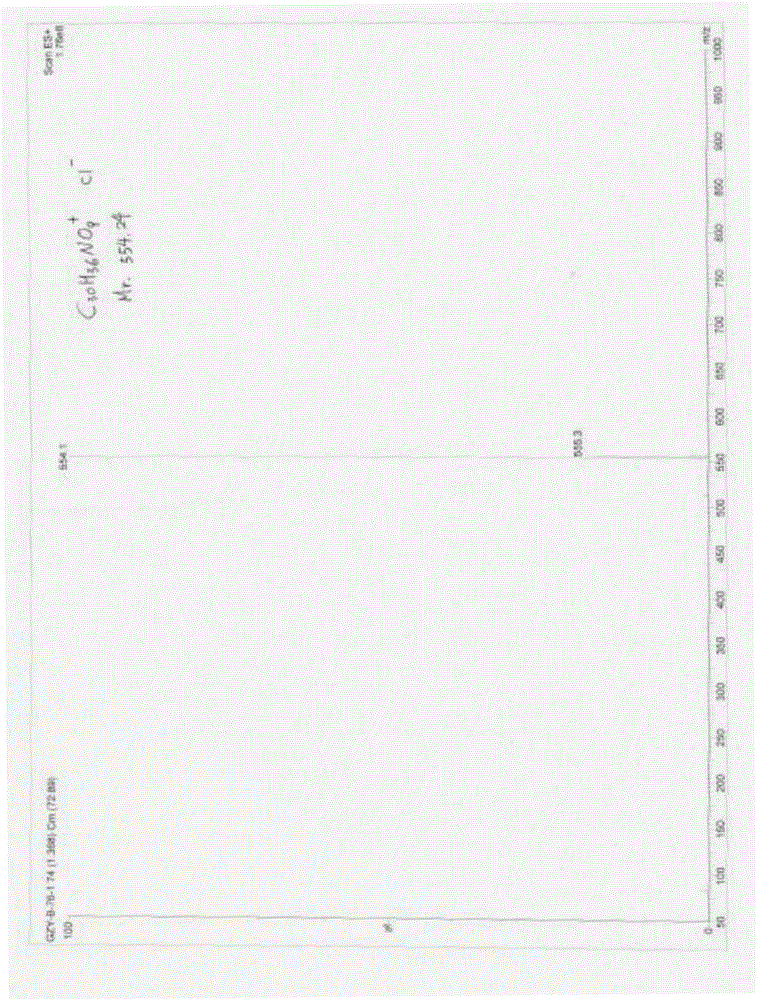
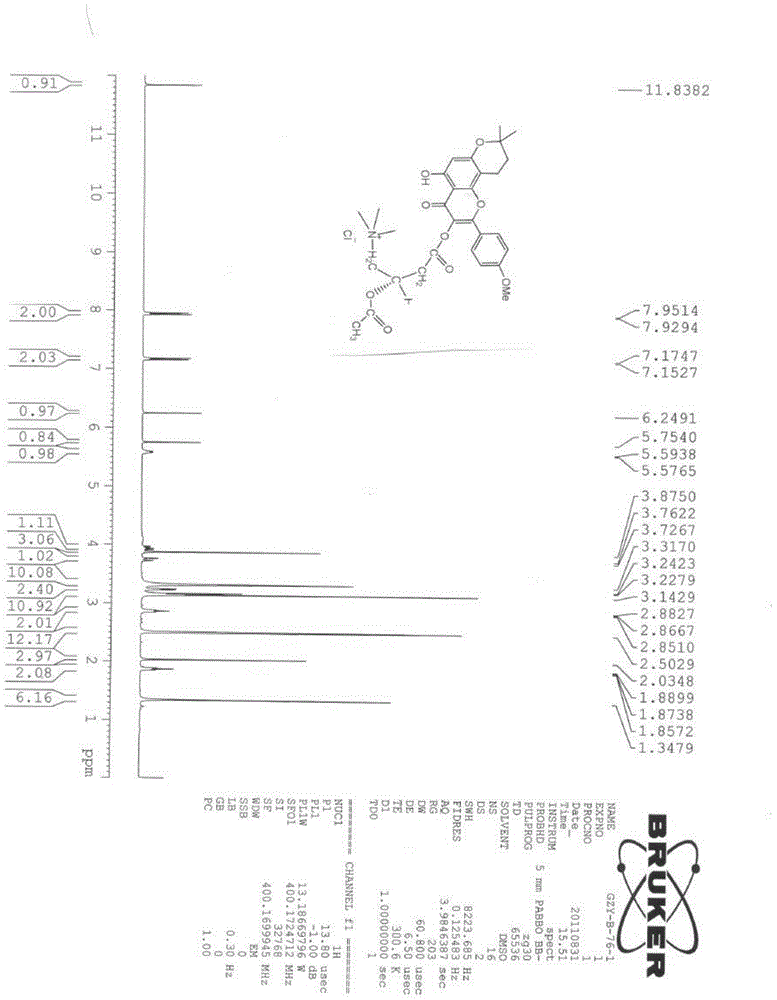
[0055] Preparation and identification of embodiment 1 formula II compound
[0056] 1. Sources of raw materials and reagents
[0057] Table 1 Sources of raw materials and reagents
[0058]
[0059]
[0060] 2, the preparation of L-carnitine acid chloride
[0061] According to the preparation method of Example 2 disclosed in Chinese Patent No. 200810014212.X, the L-carnitine acid chloride was prepared. Take 3g (0.015mol) of L-carnitine hydrochloride, add 10ml of trifluoroacetic acid, stir to dissolve, add 4.3mL of acetyl chloride in portions at 0°C, raise the temperature to 45-50°C after dropping, and keep the reaction for 20 hours. After the reaction, take it out and cool to room temperature, add 100ml of acetone, stir for 2 hours, filter, add 100ml of diethyl ether to the filtrate, stir until a white precipitate precipitates, put it in the refrigerator for 12 hours. After taking it out, stir for 1 hour, filter, and dry to obtain 5.36 g of white solid, which is acetyl-...
Embodiment 4
[0090] Preparation of formula II compound tablet
[0091] Take 50g of the compound of formula II, grind it and pass it through an 80-mesh sieve, add 50g of microcrystalline cellulose and mix evenly, add 2% hypromellose as a binder to make a soft material, granulate and dry, add a disintegrant 4% sodium carboxymethyl starch and 0.5% magnesium stearate as a lubricant, mixed evenly, and compressed into tablets. Each tablet contains 0.05g of the compound of formula II, administered orally, 3 times a day, 1 tablet each time.
Embodiment 5
[0093] The preparation of formula II compound injection
[0094] Take 100g of the compound of formula II, add an appropriate amount of water for injection to dissolve, add the prepared amount of 0.02% activated carbon, stir for 5-10min, filter, dilute the filtrate to about 10L, add sodium chloride to adjust the osmotic pressure to isotonicity, and adjust the pH to 7.5- 8.0, ultrafiltration, potting into 1000 vials (10ml / vial), sterilized, and packaged to obtain the compound injection of formula II. Injection administration, 2 times a day, 1 stick each time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com