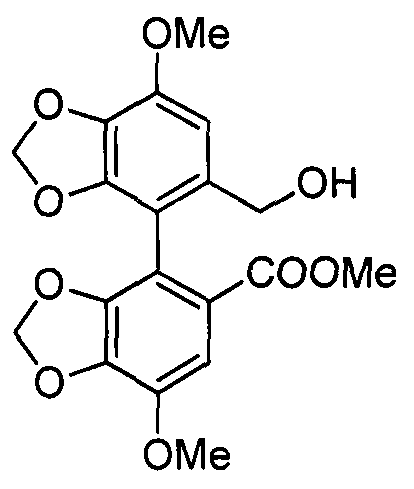
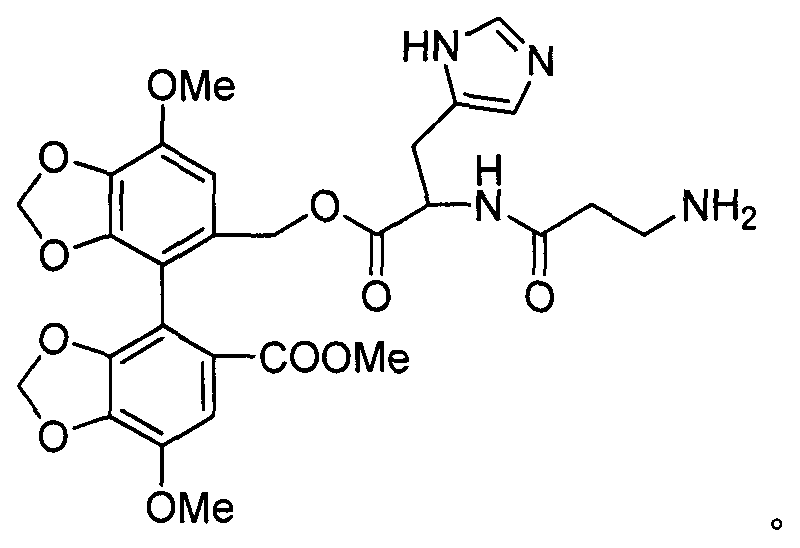
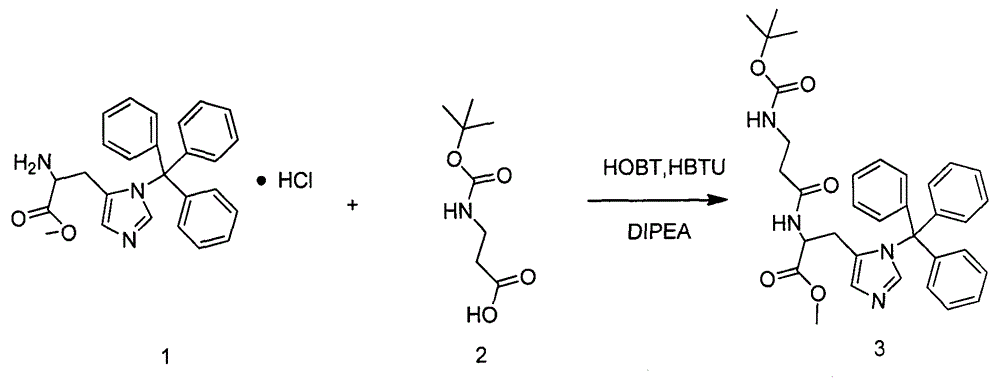
Bicyclol-carnosine conjugate, and preparation method and application thereof
A technology of bicyclol and conjugates, which is applied in the field of bicyclol-carnosine conjugates and its preparation, can solve the problems of poor antiviral ability, poor water solubility of bicyclol, and low oral utilization, and achieve liver protection and enzyme reduction Antiviral ability, good water solubility, and high oral utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of bicyclol-carnosine conjugate.
[0039] (1) Fully Protected Carnosine
[0040]
[0041]Weigh 4.482g of compound 1 (10mmol) and dissolve it in 50mL of DMF, add 10mL of triethylamine to remove hydrochloride, stir for 10min, add 2.268g of compound 2 (12mmol) under ice cooling, 1.62g of 1-hydroxybenzotriazole (HOBT ) (12mmol), 3.48mL N, N'diisopropylethylamine (DIPEA) (20mmol), after stirring for 10min, add 4.55g benzotriazole-N, N, N', N'-tetramethylurea Hexafluorophosphate (HBTU) (12mmol). Remove the ice bath and react for 4h. Add a large amount of water to the reaction solution and let it stand. After clarification, filter the supernatant, dissolve the solid with ethyl acetate, wash with 1mol / L HCl, saturated aqueous sodium bicarbonate solution, and saturated aqueous sodium chloride solution successively, and dry to obtain a white solid 4.22g, which is the fully protected carnosine 3, the HPLC analysis purity is greater than 80%, and the yiel...
Embodiment 2
[0052] Example 2 Preparation of bicyclol-carnosine conjugate.
[0053] (1) Condensation reaction between bicycloalcohol and protected histidine, the product is deprotected to obtain a bicycloalcohol-histidine conjugate;
[0054] (1.1) Conjugation of protected histidine (Fmoc-His(Trt)-OH) to bicyclic alcohol
[0055]
[0056] Weigh 2.1g of Fmoc-His(Trt)-OH (3.39mmol) and dissolve it in 20mL of dichloromethane (DCM), cool in an ice bath, add 40mg of 4-dimethylaminopyridine (DMAP) (0.328mmol) and 750mg of 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) (3.91mmol), stirred for 10min, added 1.0118g of bicyclic alcohol (2.60mmol), and reacted for 6h. Add 100 mL of dichloromethane (DCM) to the reaction solution, wash with 1 mol / L hydrochloric acid solution, saturated aqueous sodium bicarbonate solution, and saturated sodium chloride aqueous solution successively, dry, and gradient elution on a silica gel column, the mobile phase is PE:EA=4: 1-1:1, 1.58 g o...
Embodiment 3
[0071] Example 3 Preparation of bicyclol-carnosine conjugate.
[0072] (1) Condensation reaction between bicycloalcohol and protected glycine, product deprotection, to obtain glycine-bicycloalcohol conjugate;
[0073] (1.1) Conjugation of protected histidine (Fmoc-His(Trt)-OH) to bicyclic alcohol
[0074] The specific embodiment is the same as (1.1) in Example 2
[0075] (1.2) Deshistidine amino Fmoc protecting group
[0076] The specific embodiment is the same as (1.2) in Example 2
[0077] (2) Condensation reaction of histidine-bicyclol conjugate with protected β-alanine to obtain fully protected carnosine-bicyclol conjugate;
[0078] Weigh 5mmol bicyclol-histidine alcohol conjugate, 10mmol protected β-alanine Boc-β-Ala-OH, 1mmol HOBt and dissolve in 18mL DMF, cool in an ice bath, add 0.8mL DIPEA, stir for 5min , add 1mmol HBTU, react at 40°C for 4h, add 50mL ethyl acetate to the reaction liquid, wash with 1mol / L HCl, saturated aqueous sodium chloride solution successive...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com