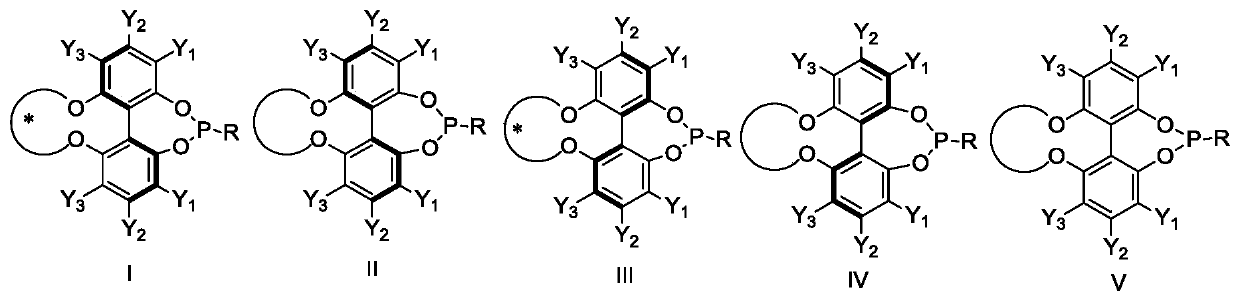
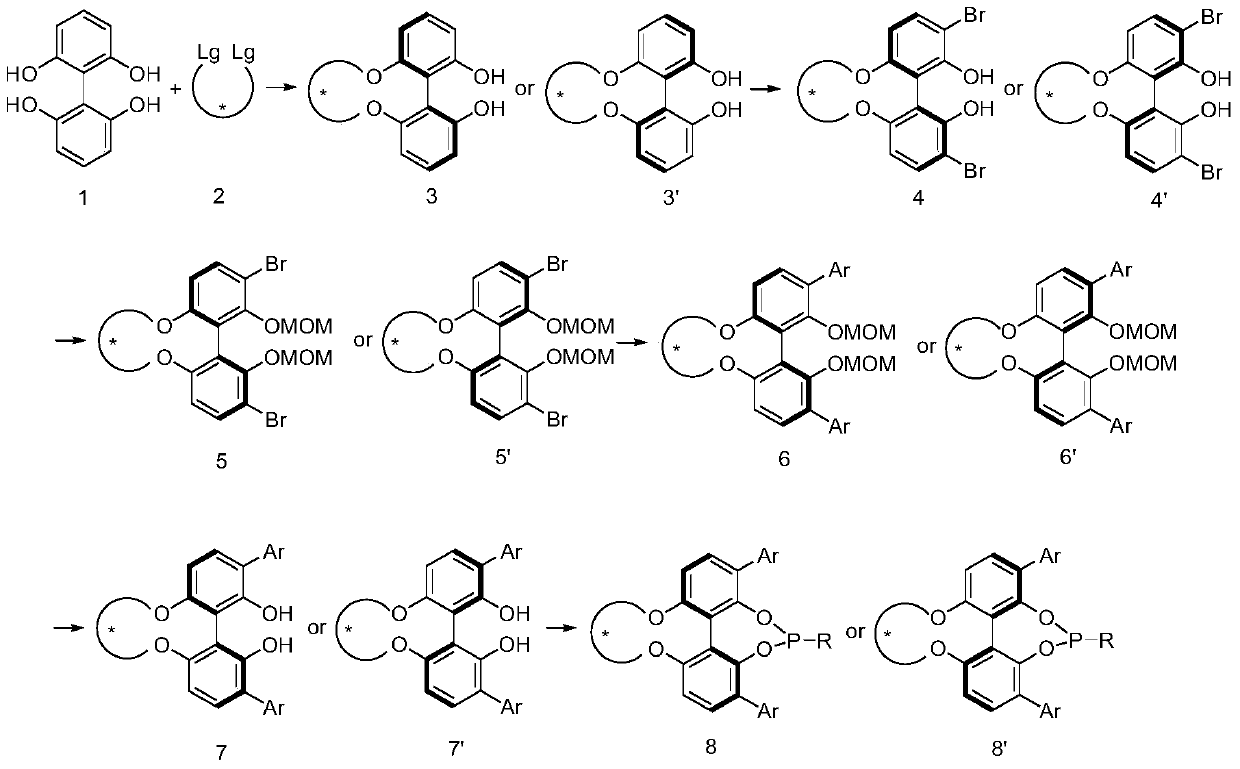
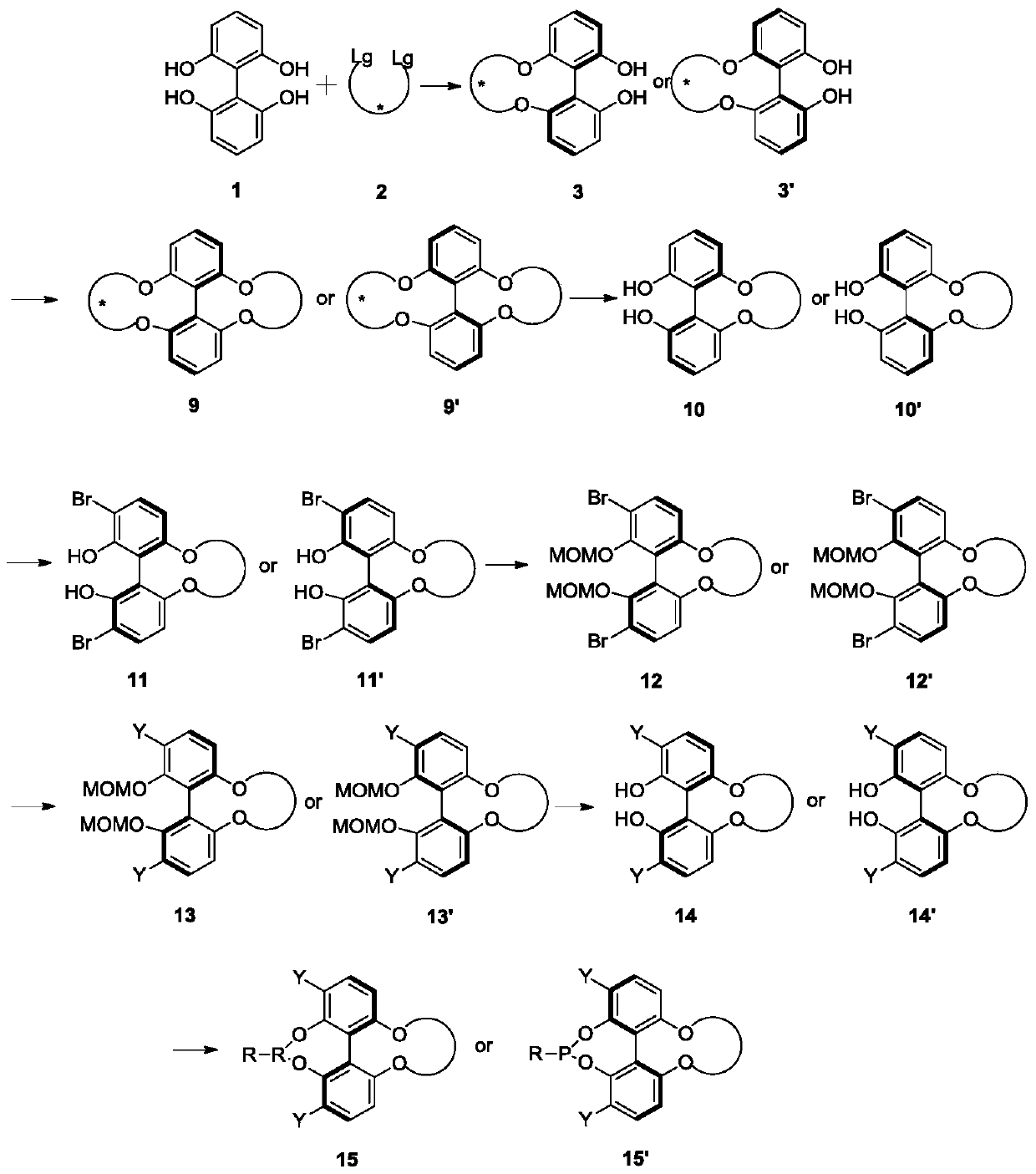
A kind of phosphoramidite ligand and its preparation method and application
A phosphoramidite, the phosphoramidite technology, applied in the direction of organic chemistry methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as lack
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 The prepared (R)-[6,6'-((S)-1,2-propylene glycol oxygen)]-2,2'-N,N-diisopropylphosphoramidite (16) is example
[0061]
[0062] Synthesis of (R)-1,2-propanediol dimesylate (2)
[0063] (R)-1,2-propanediol (1g, 13.14mmol), CH 2 Cl 2 (50mL), Et 3 N (3.66mL, 26.28mmol) system, slowly add methanesulfonyl chloride (2.03mL, 23.28mmol) dropwise in CH at 0°C 2 Cl 2 (20mL) solution, dripping over 1h, the reaction continued to stir for 1h, slowly rose to room temperature and stirred for 20h. The reaction solution was poured into 100 mL of 1N HCl solution, CH 2 Cl 2 Extraction, anhydrous Na 2 SO 4 dry. The solvent was spin-dried under reduced pressure, and the crude product was recrystallized to obtain 3.97 g of a white solid product with a yield of 67%. 1 H NMR (400MHz, CDCl 3 )δ5.14-5.09(m,1H),4.23-3.92(m,2H),3.25(s,6H),1.39(m,3H)ppm.
[0064] Synthesis of (R)-[6,6'-((S)-1,2-propanedioloxy)]-2,2'-dihydroxybiphenyl
[0065] 2,2',6,6'-Tetrahydroxybipheny...
Embodiment 2
[0072] Example 2 to prepare (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2,2'-N,N-bis[(1S)-1- Phenylethyl]phosphoramidite (17) as an example
[0073]
[0074] Synthesis of (R)-[6,6'-((S,S)-2,3-butanedioloxy)]-2,2'-dihydroxybiphenyl
[0075] 2,2',6,6'-Tetrahydroxybiphenyl (1.77g, 8.12mmol), anhydrous cesium carbonate (6.08g, 18.67mmol), anhydrous DMF (232mL), heating and stirring at 80°C, slowly drop into A solution of (R,R)-2,3-butanediol dimesylate (1.98g, 8.12mmol) in anhydrous DMF (81mL) was added dropwise over 4 hours. The reaction mixture was continued at 80 °C for 12 h. DMF was removed under reduced pressure, extracted with ethyl acetate, anhydrous Na 2 SO 4 dry. The solvent was spin-dried under reduced pressure, and the crude product was subjected to silica gel column chromatography to obtain a light yellow solid. Product yield: 1.28 g, yield: 58%. product 1 H NMR (300MHz, CDCl 3 )δ7.26-7.19(m,2H),6.78-6.71(m,4H),6.11(s,2H),3.95-3.85(m,2H),1.37(d,J=5.4Hz,6H)ppm.
...
Embodiment 3
[0078] Example 3 to prepare (S)-[6,6'-((R,R)-2,4-pentanedioloxy)]-2,2'-N,N-bis[(1R)-1- Phenylethyl]phosphoramidite (18) as an example
[0079]
[0080] Synthesis of (S)-[6,6'-((R,R)-2,4-pentanedioloxy)]-2,2'-dihydroxybiphenyl
[0081] 2,2',6,6'-Tetrahydroxybiphenyl (0.80g, 3.67mmol), anhydrous cesium carbonate (3.56g, 10.93mmol), anhydrous DMF (120mL), stirred at room temperature. A solution of (2S,4S)-pentanediol p-toluenesulfonate (1 g, 2.43 mmol) in anhydrous DMF (40 mL) was added dropwise, and the solution was completed after 4 hours. Continue to react at room temperature for 120h. DMF was removed under reduced pressure, extracted with ethyl acetate, anhydrous Na 2 SO 4 dry. The solvent was spin-dried under reduced pressure, and the crude product was subjected to silica gel column chromatography to obtain 0.69 g of a light yellow solid product with a yield of 68%. 1 HNMR (400MHz, CDCl 3 )δ7.20-7.14(m,2H),6.72(d,J=8.1Hz,2H),6.65-6.58(m,2H),4.69-4.57(m,2H),1.36(d,J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com