Novel methyl benzoylformate initiator and preparation method thereof
A technology of benzoylformic acid and an initiator, which is applied in the field of reactive benzoylformate initiators and their preparation, can solve the problems of unpleasant production process, high initiation effect, high cost, etc. Good effect, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
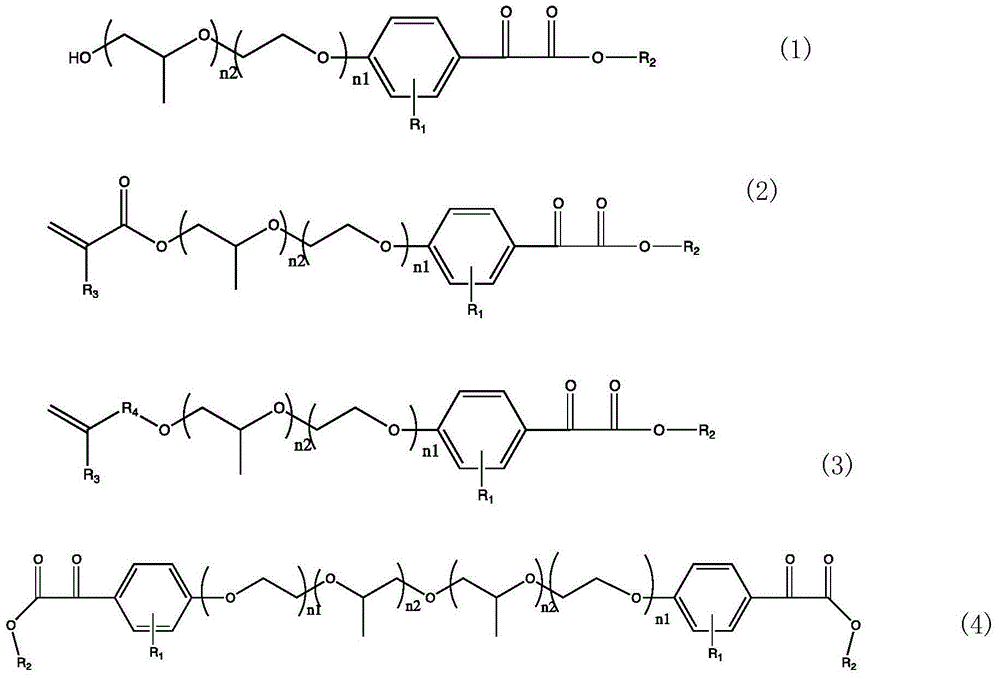
[0047] 4, a kind of preparation method of novel benzoyl formate initiator (1) as claimed in claim 1, is characterized in that: comprise the following steps:
[0048] (1) Add phase transfer catalyst, substituent phenol and glyoxylic acid into water and methylene chloride in the reaction vessel, stir, and add sodium hydroxide aqueous solution dropwise at a certain temperature. After the dropwise addition, continue to react for a period of time, add water to let The solid is dissolved, the organic phase is separated, the aqueous phase is acidified with acid, and then extracted and washed with ethyl acetate or ether;
[0049]
[0050] ⑵When peroxide or bromine is used as an oxidant to catalyze the oxidation of aromatic hydrocarbons to synthesize aromatic ketones, and esterify with alcohols to form esters
[0051]
[0052] (3) The product of compound (5) reacts with ethylene oxide and propylene oxide to obtain the target product compound (1).
[0053]
[0054]In the step ...
Embodiment 1
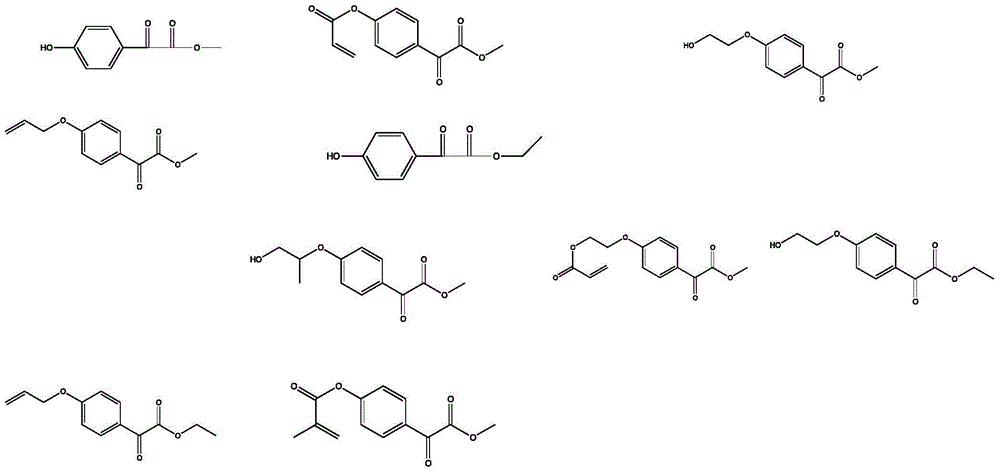
[0076] The preparation of 4-hydroxybenzoylformic acid methyl ester, concrete method is as follows:
[0077]
[0078] (1) Put 9.4g of phenol, 6.7g of glyoxylic acid, 50ml of dichloromethane, and 0.9g of tetrabutylammonium bromide into a 100mL four-necked flask under nitrogen protection, and add 24g of 50% sodium hydroxide aqueous solution dropwise under rapid stirring to control The temperature is 0-10°C, HPLC or GC monitors the reaction until the reaction is completed, then cools down to room temperature, extracts the product with ethyl acetate, concentrates under reduced pressure, washes with water 1-3 times, and separates the organic phase;
[0079] (2) Add 8g of 30% hydrogen peroxide dropwise and oxidize at 40°C. Extract with 50ml of toluene to obtain the organic phase;
[0080] (3) 2.5 g of methanol and 1.4 g of p-toluenesulfonic acid were added to the organic phase for esterification at 110° C., desolvated and crystallized to obtain 15.3 g of methyl 4-hydroxybenzoylfo...
Embodiment 2
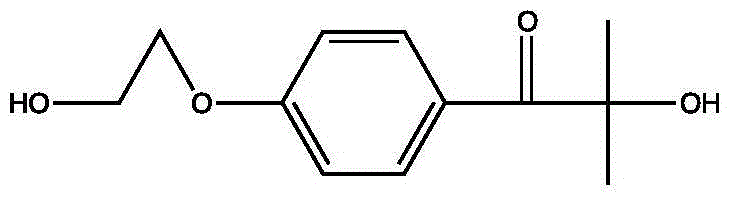
[0082] The preparation of 4-(2-hydroxyethoxy) methyl benzoylformate, the steps are as follows:
[0083]
[0084] (1) Put 100ml of toluene and 15.3g of methyl 4-hydroxybenzoylformate into a 250mL four-necked flask under the protection of nitrogen, slowly introduce ethylene oxide, stir and heat to 70°C, keep the temperature for about 2 hours, and use The reaction was monitored by thin-layer chromatography or liquid phase. After the reaction was complete, the temperature was lowered to room temperature, and the solvent was removed.
[0085] (2) The residue was diluted with 100ml of dichloromethane, washed with 100ml of water, dried with anhydrous sodium sulfate, desolvated, and purified by distillation under reduced pressure to obtain 17.5g of a colorless oil with a yield of 92.0% and a purity of 99.2%.
[0086] Proton nuclear magnetic resonance spectrum 1HNMR (CDCl3, ppm unit): 8.11 (s, 2H), 7.18 (s, 2H), 4.33 (s, 2H), 3.69 (s, 2H), 3.68 (s, 3H), 3.65 (s , 1H) C NMR spectrum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com