Homogenous precipitation method for preparing Fe2O3 nanobelt and Fe2O3 nanobelt-carbon composite material
A technology of carbon composite materials and composite materials, which is applied in the field of uniform precipitation of composite materials, can solve the problems of poor cycle performance and poor rate performance of negative electrode materials, and achieve the effects of low self-discharge rate, high working voltage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] First, take 0.5g FeCl 3 ·6H 2 O and 0.134 g of sodium oxalate were sequentially dissolved in 200 mL of deionized water. Take 0.20 g of ascorbic acid and dissolve it in about 5 mL of deionized water, then add the ascorbic acid aqueous solution dropwise to the stirring solution above, and continue to stir for about 3 hours after the addition is complete. After centrifugal separation, the product was washed three times with deionized water, and then dried in a vacuum oven at 85° C. to obtain a yellow product. The obtained yellow product was placed in a porcelain boat and heat-treated at 700°C for 3 hours in an air atmosphere to obtain the final product Fe 2 o 3 nanobelt.
Embodiment 2
[0037] First, take 25.0 mg of carbon nanotubes (CNTs), add it into 200 mL of deionized water, and stir it ultrasonically for 3 h. Then, take 6.06g Fe(NO 3 ) 3 9H 2 O and 6.6g of ammonium oxalate were sequentially added to the above dispersion of CNTs and stirred to dissolve them. Get 3.52g of sodium hypophosphite and dissolve it in about 10mL of deionized water, then add the aqueous solution of sodium hypophosphite dropwise to the stirring Fe(NO 3 ) 3 , ammonium oxalate and CNTs mixture, continue to stir for about 0.5h after the dropwise addition. After centrifugation, the product was washed three times with ethanol, and then dried in a vacuum oven at 85°C to obtain a black product. The obtained black product was placed in a porcelain boat and heat-treated at 250°C for 3 hours in an air atmosphere to obtain the final product Fe 2 o 3 -CNTs composites.
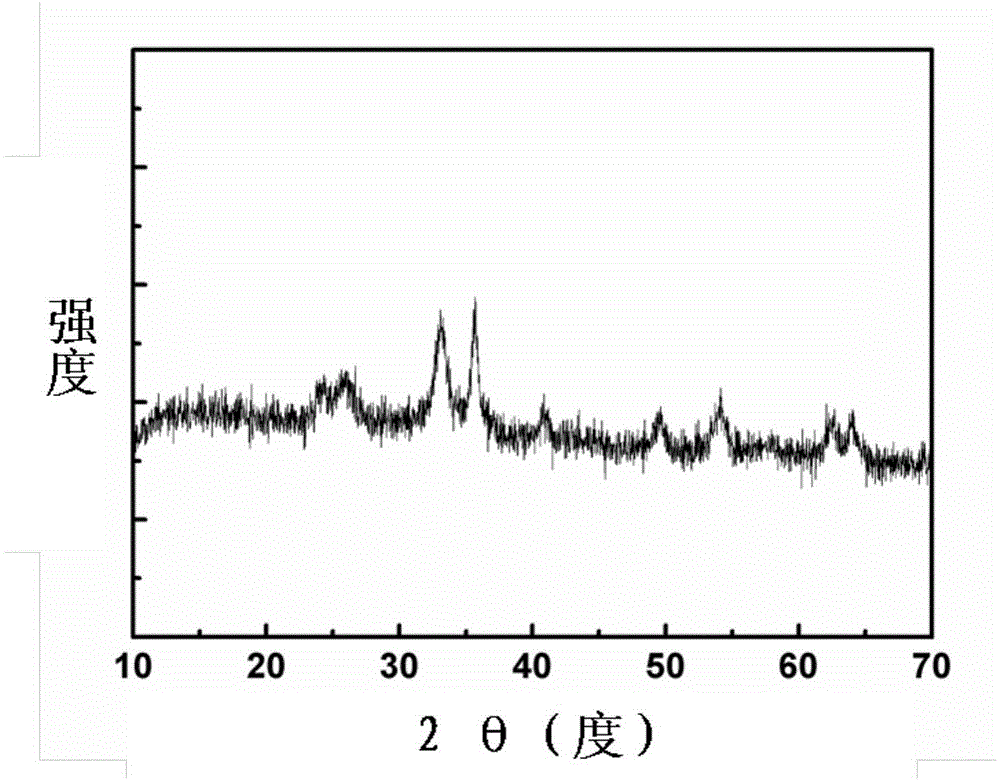
[0038] figure 1 For the prepared Fe 2 o 3 / Carbon XRD pattern, it can be seen from the figure that the diffraction...
Embodiment 3
[0040] First, take 100 mg of CNTs, add it into 200 mL of deionized water, and disperse for 0.5 h with ultrasonic-assisted stirring. Then, take 0.5g FeCl respectively 3 ·6H 2 O and 0.134g of sodium oxalate were sequentially added to the above CNTs dispersion and stirred to dissolve it. Dissolve 0.20 g of ascorbic acid in about 5 mL of deionized water, then add the aqueous solution of ascorbic acid dropwise to the stirring FeCl 3 , sodium oxalate and CNTs mixture, after the dropwise addition, continue to stir and react for about 3h. After centrifugation, the product was washed three times with ethanol, and then dried in a vacuum oven at 85°C to obtain a black product. The obtained black product was placed in a porcelain boat, heat-treated at 300°C for 0.5h in an air atmosphere, and then heat-treated at 700°C for 3h under an argon or nitrogen atmosphere to obtain the final product Fe 2 o 3-CNTs composites.
[0041] figure 2 For the prepared Fe 2 o 3 TEM image of nanobel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com