Medicine for treating chronic hepatitis B virus infection
A technology for hepatitis B virus and hepatitis B, applied in antiviral agents, drug combinations, pharmaceutical formulas, etc., to achieve non-immunogenicity, safe and convenient use, and reduce liver lymphocyte infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1, Purification of Pleurotus lectin
[0073] 1. After the fruiting bodies of Pleurotus pachyrhiza are mashed, the fruiting bodies of Pleurotus pachyrhiza are obtained, and 750 grams of Pleurotus pleurotus fruiting body powder are prepared by using 2000 milliliters of 0.15mM NaCl aqueous solution at 4°C (0.1-0.2mM NaCl aqueous solution at 0-4°C) Both) were extracted for 2-12 hours, and the supernatant was taken after centrifugation. (The ratio of the fruiting bodies of Pleurotus chinensis to the aqueous solution of NaCl is 500-750 grams: 2000 milliliters is acceptable)
[0074] 2. The supernatant was washed with 80% saturation (NH 4 ) 2 SO 4 The aqueous solution (30%-80% saturation is acceptable) is precipitated for 6 hours (4-8 hours are acceptable), and the precipitate is collected after centrifugation.
[0075] 3. Dissolving the precipitate with PBS and then dialysis to obtain a crude sample of Pleurotus agglutinin.
[0076] 4. The crude sample of Pleurot...
Embodiment 2
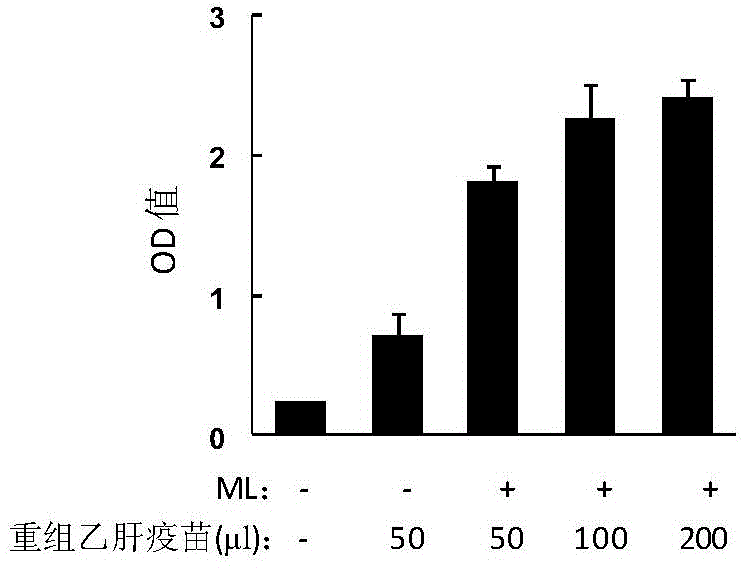
[0083] Example 2. Effect of Pleurotus agglutinin on the Effect of Recombinant Hepatitis B Vaccine in Treating Hepatitis B Virus Infection
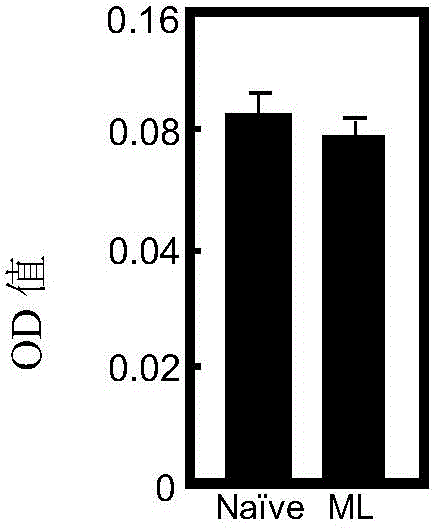
[0084] 1. Detection of immunogenicity and side effects of Pleurotus agglutinin
[0085] (1) Immunization
[0086] Twenty female C57BL / 6 mice were randomly divided into 2 groups, 10 in each group, and were treated as follows:
[0087] Control group: Each mouse was injected with 100 microliters of normal saline as a control.
[0088] Immunization group: each mouse was injected with 1 microgram of Pleurotus agglutinin prepared in Example 1 dissolved in physiological saline in 100 microliters.
[0089] The mice in each of the above groups were immunized by intramuscular injection for a total of 3 times, with an interval of 2 weeks between each immunization, and the immunization time points were consistent.
[0090] (2) Detection of Pleurotus agglutinin-specific antibody
[0091] On the 7th day after the last immunization, blood was taken fro...
Embodiment 3
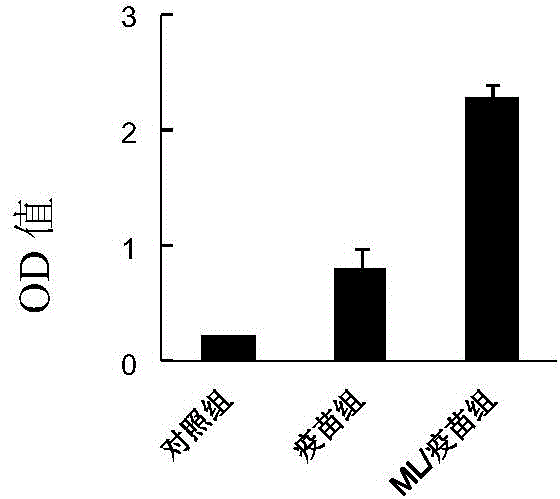
[0159] Example 3. Comparison of therapeutic effects of Pleurotus agglutinin and HBV DNA vaccine synergy and Pleurotus agglutinin and recombinant hepatitis B vaccine on HBsAg transgenic mice
[0160] 1. Immunity
[0161] Twenty HBsAg transgenic mice were randomly divided into 4 groups, with 5 mice in each group, and were treated as follows:
[0162] 1) Model group: each transgenic mouse was injected with 100 microliters of normal saline as a control;
[0163] 2) Pleurotus agglutinin combined with HBV DNA vaccine group: 100 micrograms of proVAX-S2 plasmid and 1 microgram of Pleurotus agglutinin prepared in Example 1 were added to 100 microliters of normal saline to make an immune reagent, and each mouse was injected 100 μl of the immunological reagent.
[0164] 3) Pleurotus agglutinin synergistically with recombinant hepatitis B vaccine group: add 1 microgram of Pleurotus agglutinin prepared in Example 1 to 100 microliters of recombinant hepatitis B vaccine (containing 2 micro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com