A kind of preparation method for the magnetic target drug that is used to inhibit the growth of Hela cell
A magnetic and cellular technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of cumbersome preparation process of magnetic composite carriers, lack of consideration of the characteristics of magnetic composite carriers, etc. Inhibition of tumor cell growth, satisfactory particle size, good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
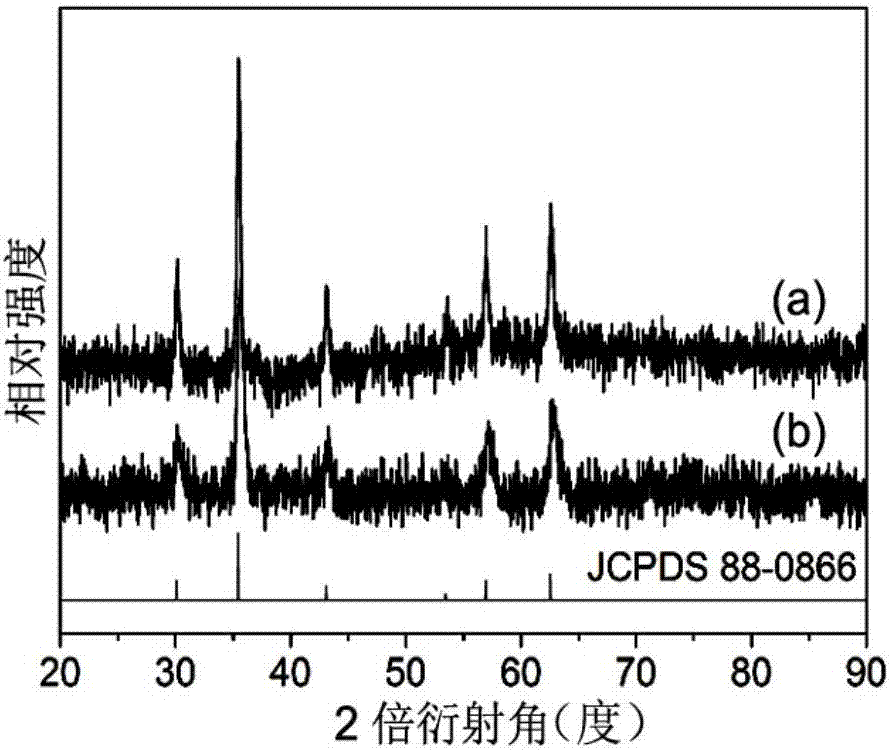
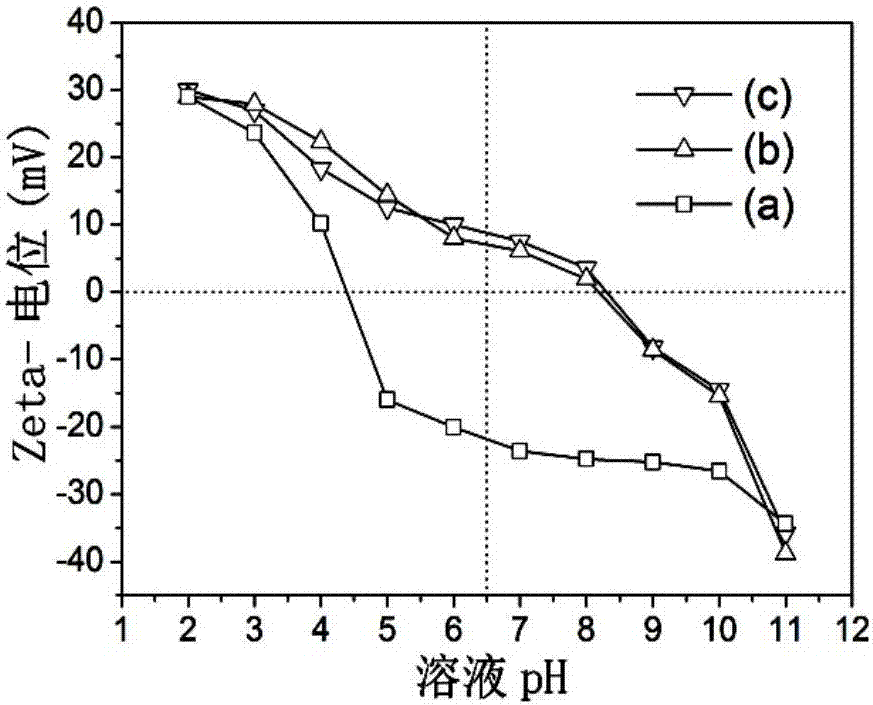
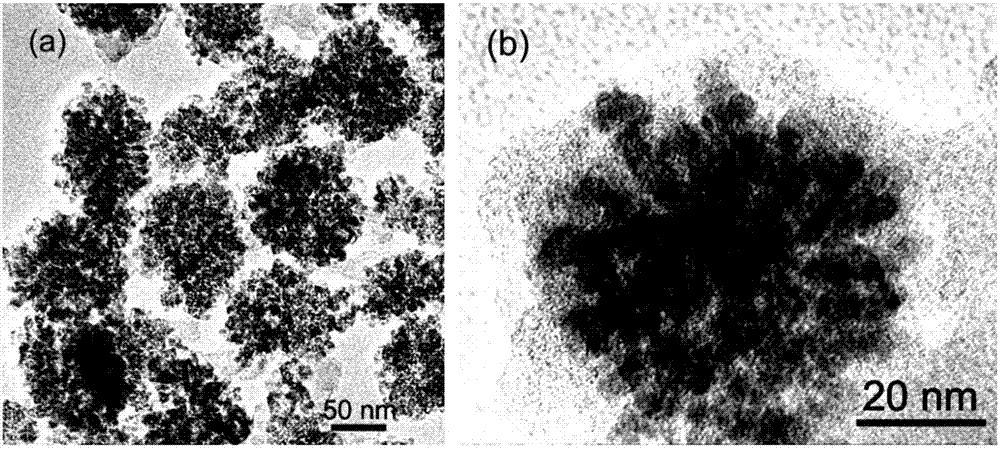
Image
Examples
Embodiment 1
[0055] A preparation method of a magnetic target drug for inhibiting HeLa cell growth, the steps are as follows:
[0056] a. Dissolve 1g of iron acetylacetonate in 70ml of ethylene glycol, add 0.7g of anhydrous sodium acetate and 0.5g of polyethylene glycol 4000, and stir vigorously (300r / min) for 0.5h to obtain a mixed solution A;
[0057] b. Transfer the mixed solution A to a 100mL autoclave lined with polytetrafluoroethylene, raise the temperature to 190°C, keep the temperature for 20 hours, and lower the temperature to room temperature to obtain the mixed solution B; the solid in the mixed solution B is subjected to magnetic Separation, retain the solid, wash the solid with water until the aqueous solution is neutral, and magnetically separate to obtain solid C;
[0058] c. Add 40mL of 0.1mol / L hydrochloric acid aqueous solution to the above solid C, ultrasonically disperse for 0.5h, magnetically separate, keep the solid, add 20mL of ethanol solution in which 0.2mL of pyrr...
Embodiment 2
[0064] A preparation method of a magnetic target drug for inhibiting HeLa cell growth, the steps are as follows:
[0065] a. Dissolve 2 g of iron acetylacetonate in 80 mL of ethylene glycol, add 4.62 g of sodium acetate trihydrate and 0.5 g of polyethylene glycol 8000, and stir vigorously (400 r / min) for 1 hour to obtain a mixed solution A;
[0066] b. Transfer the mixed solution A to a 95mL autoclave lined with polytetrafluoroethylene, raise the temperature to 200°C, keep the temperature for 36 hours, and lower the temperature to room temperature to obtain the mixed solution B; Magnetic separation, retaining the solid, washing the solid with water until the aqueous solution is neutral, magnetic separation, to obtain solid C;
[0067]c. Add 40 mL of 0.5 mol / L hydrochloric acid aqueous solution to the above solid C, ultrasonically disperse for 1 h, magnetically separate, and leave the solid, add 20 mL of ethanol solution in which 0.6 mL of pyrrole (processed by vacuum distillat...
Embodiment 3
[0080] The application of the above-mentioned magnetic target drug in inhibiting the growth of HeLa cells, the steps are as follows:
[0081] (1) get 0.1g embodiment 2 preparation magnetic target drug 50mL volume concentration is 75% alcohol sterilization 24h, then the magnetic target drug after the sterilization is dispersed in 10mL pH is in the phosphate buffered saline solution (PBS) of 7.4, spare;
[0082] (2) Set the concentration to 1.0×10 5 / mL Hela cell suspension was inoculated in a 96-well plate, 100 μL / well of DMEM medium was added, and the 2 Cultivate under conditions for 24 hours, discard the supernatant, add new DMEM medium and magnetic target drug, the concentration of the magnetic target drug in the medium is controlled at 0.1, 0.2, 0.3, 0.4 and 0.5 mg / mL; each group sets 5 In addition, a negative control group and a toxicity control group were set up, wherein Fe in the toxicity control group 3 o 4 , Fe 3 o 4 The concentration of / PPy is 0.44mg / mL, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com