Method for preparing anisic aldehyde
A technology of p-methoxybenzaldehyde and p-hydroxybenzaldehyde, which is applied in the field of preparation of p-methoxybenzaldehyde, can solve the problems of low yield and unsuitability for large-scale industrial production, and achieve the effect of improving conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
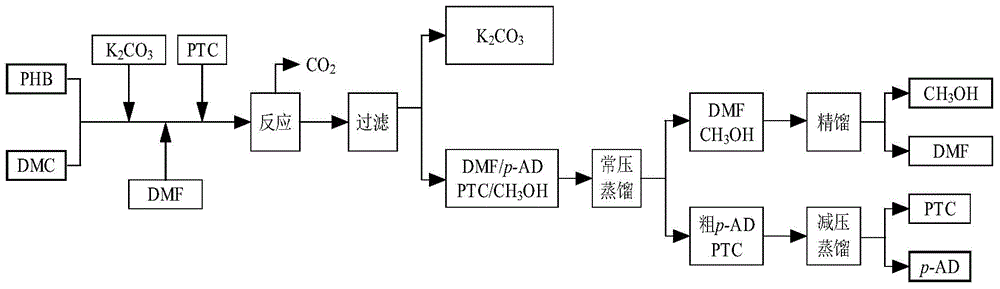
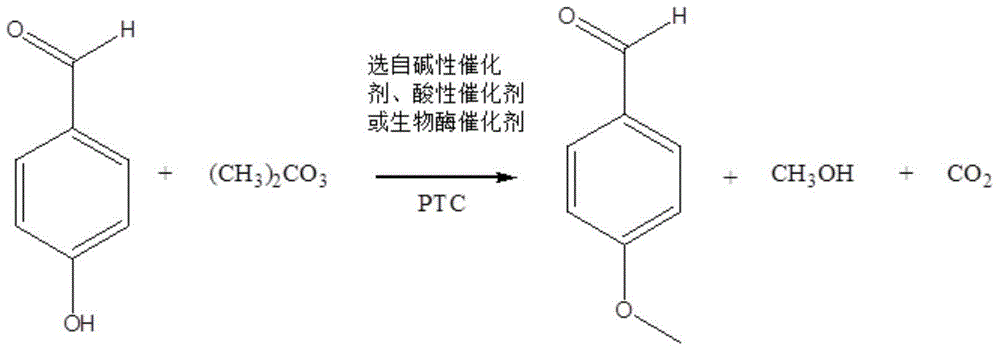
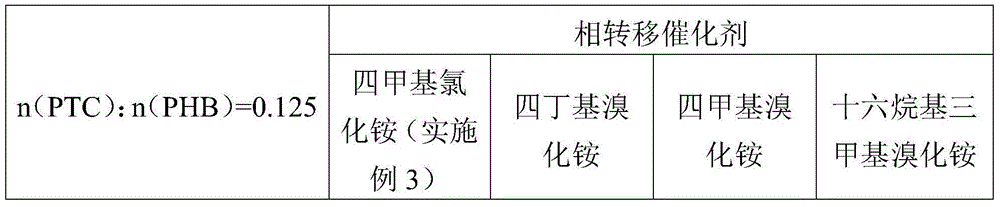
[0039] Take 0.05mol of p-hydroxybenzaldehyde and place it in a 250ml four-necked flask with a stirrer and a reflux device, add 20g of DMF, 0.005mol of tetramethylammonium chloride, and 0.0375mol of potassium carbonate; Add 0.124mol dimethyl carbonate, after the dropwise addition, heat up to 120°C and reflux for 3h to complete the reaction. After the reaction is completed, filter the reaction solution to recover potassium carbonate, recover the solvent by atmospheric distillation, and collect the 500 Pa, 140-150 ° C fraction by vacuum distillation at the bottom of the bottle to obtain the product p-methoxybenzaldehyde. DMF and by-product methanol are separated by rectification, and the tetramethylammonium chloride that has not participated in the reaction at the bottom of the bottle is recovered by recrystallization using by-product methanol.
[0040]Through analysis and calculation, the reaction yield is 88%, and the product purity is 98.84%.
Embodiment 2
[0042] Take 0.1mol of p-hydroxybenzaldehyde and place it in a 250ml four-neck flask equipped with a stirrer and reflux device, add 40g of DMF, 0.01mol of tetramethylammonium chloride, and 0.075mol of potassium carbonate; Add 0.124mol dimethyl carbonate, after the dropwise addition, heat up to 150°C and reflux for 5h to complete the reaction. After the reaction is completed, filter the reaction solution to recover potassium carbonate, recover the solvent by atmospheric distillation, and collect the 500 Pa, 140-150 ° C fraction by vacuum distillation at the bottom of the bottle, which is the product p-methoxybenzaldehyde. DMF and by-product methanol are separated by rectification, and the tetramethylammonium chloride that has not participated in the reaction at the bottom of the bottle is recovered by recrystallization using by-product methanol.
[0043] Through analysis and calculation, the reaction yield is 84%, and the product purity is 99.32%.
Embodiment 3
[0045] Take 0.075mol of p-hydroxybenzaldehyde and place it in a 250ml four-necked flask with a stirrer and a reflux device, add 30g of DMF, 0.009375mol of tetramethylammonium chloride, and 0.056mol of potassium carbonate; Add 0.124mol dimethyl carbonate, after the dropwise addition, heat up to 130°C and reflux for 5h to complete the reaction. After the reaction is completed, filter the reaction solution to recover potassium carbonate, recover the solvent by atmospheric distillation, and collect the 500 Pa, 140-150 ° C fraction by vacuum distillation at the bottom of the bottle, which is the product p-methoxybenzaldehyde. DMF and by-product methanol are separated by rectification, and the tetramethylammonium chloride that has not participated in the reaction at the bottom of the bottle is recovered by recrystallization using by-product methanol.
[0046] Through analysis and calculation, the reaction yield is 93%, and the product purity is 97.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com