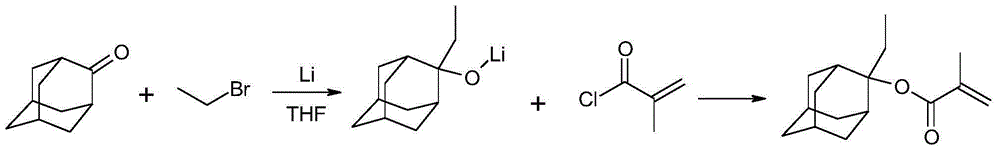
Preparation method for 2-ethyl-2-adamantanol methacrylate
A technology of adamantanol methacrylate and methacryloyl chloride, applied in the preparation of carboxylic acid halides, organic chemistry, etc., can solve the problems of complicated separation process, obtaining pure products, difficult cost control, etc., and achieve simple separation process , high product purity and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]Step 1. Dissolve 500g of adamantanone in 1550ml of anhydrous tetrahydrofuran, add 500g of bromoethane under stirring, and stir until completely dissolved under nitrogen protection.
[0065] Step 2. Add 50g of lithium metal and 450ml of anhydrous tetrahydrofuran (water content less than 200ppm) into a 5000ml reaction bottle with mechanical stirring, and drop the temperature to -5°C to 0°C while stirring, and start adding the above mixed solution for about 7 hours. After finishing, control the temperature at 10°C to 20°C.
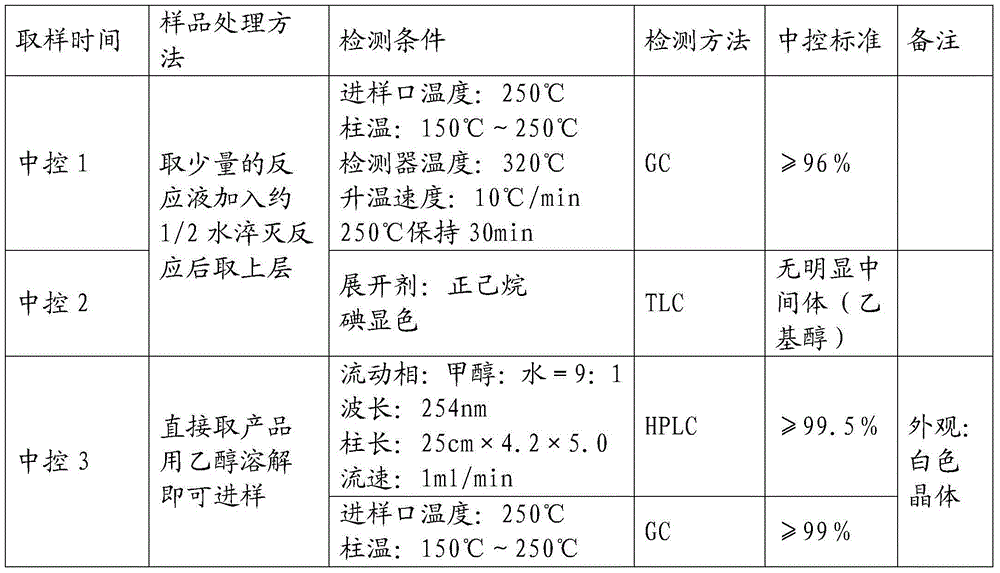
[0066] Step 3. After dripping, keep warm for 1 hour, then remove the cooling equipment and naturally rise to room temperature (20°C-25°C) and stir for about 14 hours. After sampling, add water to quench the reaction, TLC, and send the sample to GC after passing the test (central control 1).
[0067] Step 4: After cooling down to -10°C to -8°C, add 5g of phenothiazine and start dropping 418g of methacryloyl chloride to control the rate of addition and co...
Embodiment 2
[0074] Step 1. Dissolve 150g of adamantanone in 370ml of anhydrous benzene, add 120g of ethyl bromide under stirring, and stir until completely dissolved under nitrogen protection.
[0075] Step 2: Add 38.4g of magnesium metal and 190ml of anhydrous benzene into a 1000ml reaction bottle with mechanical stirring, and drop the temperature to -5°C to 0°C while stirring, start adding the above mixed solution dropwise for about 5 hours, and control the temperature Between 10°C and 20°C.
[0076] Step 3. After dripping, keep warm for 1 hour, then remove the cooling equipment and naturally rise to room temperature (20°C-25°C) and stir for about 12 hours. After sampling, add water to quench the reaction, TLC, and send the sample to GC after passing the test (central control 1).
[0077] Step 4: After cooling down to -10°C to -8°C, add 4.5g of 1,4-naphthoquinone and start adding 166g of methacryloyl chloride dropwise to control the rate of addition and control the temperature at -10°C ...
Embodiment 3
[0084] Step 1. Dissolve 150g of adamantanone in 300ml of anhydrous tetrahydrofuran, add 196g of bromoethane under stirring, and stir until completely dissolved under nitrogen protection.
[0085] Step 2. Add 17.5g of lithium metal and 60ml of anhydrous tetrahydrofuran into a 1000ml reaction bottle with mechanical stirring, and drop the temperature to -5°C to 0°C while stirring, and start adding the above mixed solution dropwise for about 10 hours. Control the temperature Between 10°C and 20°C.
[0086] Step 3. After dripping, keep warm for 1.5 hours, then remove the cooling equipment and naturally rise to room temperature (20°C-25°C) and stir for about 10 hours. After sampling, add water to quench the reaction, TLC, and send the sample to GC after passing the test (central control 1).
[0087] Step 4: After cooling down to -10°C to -8°C, add 2.5g of phenothiazine and start to drop 104g of methacryloyl chloride to control the rate of addition and control the temperature at -10°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com