Polycarboxyl silicon dioxide nanoparticles and preparation method thereof
A technology of hydroxy silica and nanoparticles, which is applied in the treatment of dyed organic silicon compounds, dyed low-molecular organic compounds, and fibrous fillers. The effect of high content, mild reaction conditions, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
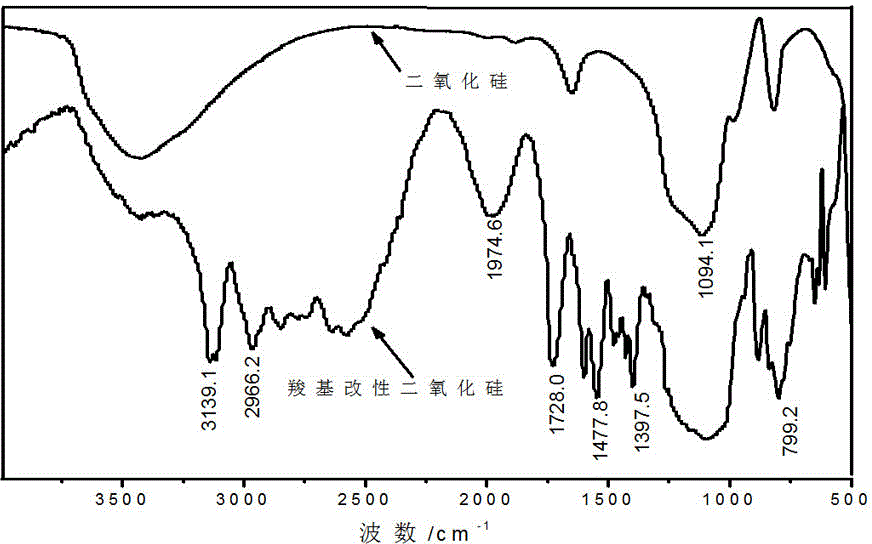
[0027] (1) Hydroxy silica nanoparticles surface treatment
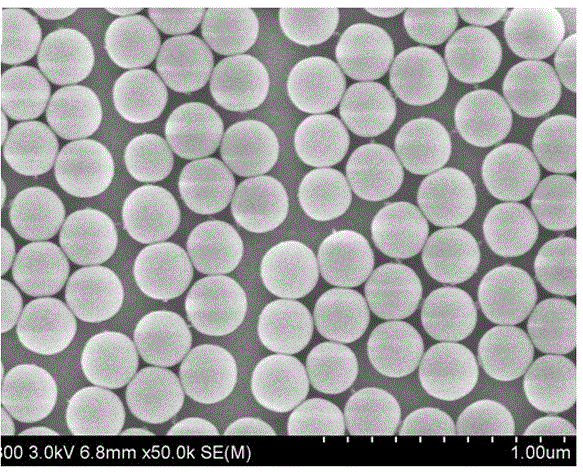
[0028] Add 100 g of tetrahydrofuran (THF) and 10 g of hydroxy silica nanoparticles (commercially available) with a particle size of 300 nm in a 1000 mL three-necked flask, and ultrasonically disperse the suspension for 10 min, then add 22.1 g of 3-aminopropyl triethoxy A solution of silane (APTES) dissolved in 20 g of tetrahydrofuran. After the mixture was refluxed at 60°C for 6 hours, the reaction was stopped, cooled to room temperature, centrifuged, and dried at 90°C for 1 hour to obtain amino silica nanoparticles.
[0029] (2) Carboxylation treatment
[0030] Add 30 g of tetrahydrofuran and 23.4 g of butane tetracarboxylic acid to a 500 mL three-necked flask in sequence, under nitrogen protection, dropwise add 16.2 g N,N' - A solution formed by dissolving carbonyldiimidazole (CDI) in 50 gTHF, and the dropping time was controlled within 1.5 hours. After the addition, react at room temperature for 5 h, then add t...
Embodiment 2
[0038] (1) Hydroxy silica nanoparticles surface treatment
[0039]Add 150g of tetrahydrofuran (THF) and 10g of hydroxylated silica nanoparticles with a particle size of 50nm in a 1000mL three-necked flask. The suspension is ultrasonically dispersed for 15min, and then 26.5g of aminoethylaminopropyltriethoxysilane is added and dissolved in 20g solution in tetrahydrofuran. After the mixture was refluxed at 65°C for 5 hours, the reaction was stopped, cooled to room temperature, centrifuged, and dried at 92°C for 1 hour to obtain amino silica nanoparticles.
[0040] (2) Carboxylation treatment
[0041] Add 50 g tetrahydrofuran and 46.8 g butane tetracarboxylic acid successively to a 500 mL three-necked flask, and under nitrogen protection, add 32.4 g N,N' - A solution formed by dissolving carbonyldiimidazole (CDI) in 70 gTHF, and the dropping time was controlled within 2 hours. After the addition, react at room temperature for 5 h, then add the above-mentioned aminoethylaminop...
Embodiment 3
[0051] (1) Hydroxy silica nanoparticles surface treatment
[0052] Add 120g of 1,4-dioxane and 8 g of hydroxylated silica nanoparticles with a particle size of 100nm in a 1000mL three-necked flask, and ultrasonically disperse the suspension for 15min, then add 26.5g of aminoethylaminopropyltrimethoxysilane A solution formed by dissolving in 25 g of 1,4-dioxane. After the mixture was refluxed at 58°C for 5 hours, the reaction was stopped, cooled to room temperature, centrifuged, and dried at 90°C for 1 hour to obtain amino silica nanoparticles.
[0053] (2) Carboxylation treatment
[0054] Add 60 g of 1,4-dioxane and 46.8 g of butane tetracarboxylic acid in sequence to a 500 mL three-necked flask, and add 31.9 g of N,N' - A solution formed by dissolving carbonyldiimidazole (CDI) in 65 g of 1,4-dioxane, and the dropping time was controlled within 1.5 hours. After the addition, react at room temperature for 5 h, then add the above-mentioned aminoethylaminopropyl silica nanopa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com