Pidotimod inhalation powder aerosol and preparation method thereof
A technology for inhaling powder sprays and pidotimod, which is applied in the fields of pharmaceutical formulations, antiviral agents, respiratory diseases, etc., can solve the problems of low absolute bioavailability, avoid adverse reactions, eliminate pain, and avoid the first over effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
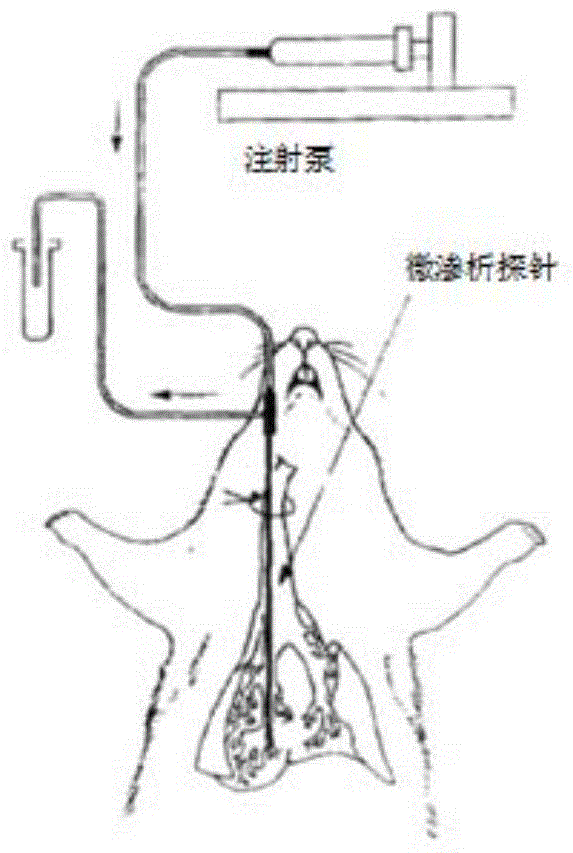
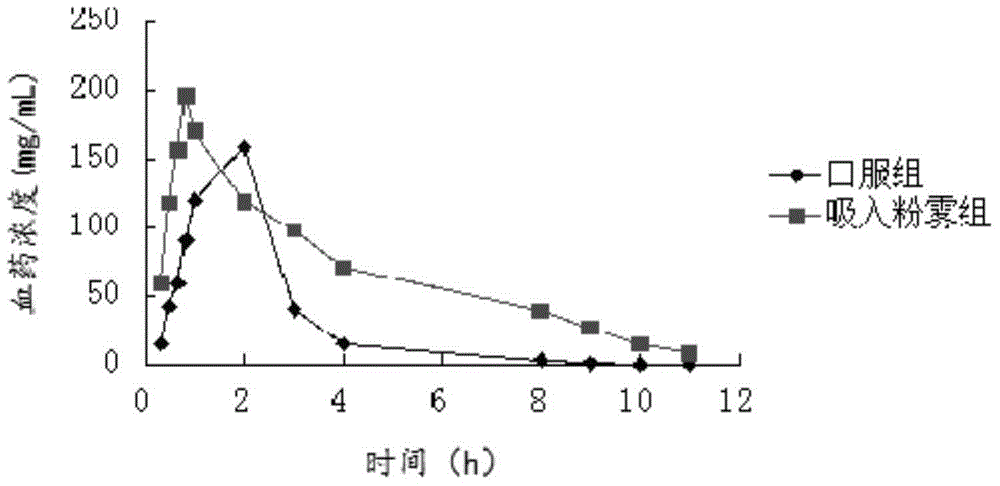
Image
Examples
Embodiment 1
[0052] Embodiment 1: Preparation of pidotimod inhalation powder aerosol
[0053] Use an ultrafine pulverizer to pulverize pidotimod to obtain an ultrafine dry powder with a particle diameter of 1 μm to 5 μm: grind and pulverize lactose with a high-speed grinding pulverizer to obtain a micropowder with a particle size of 60 μm to 80 μm; weigh 200 g Mix pidotimod micropowder with 200g lactose micropowder, fill No. 1 capsule, each filling 400mg, make 1000 capsules, and get ready.
Embodiment 2
[0054] Embodiment 2: Preparation of pidotimod inhalation powder aerosol
[0055] Use an ultrafine pulverizer to pulverize pidotimod to obtain an ultrafine dry powder with a particle diameter of 1 μm to 5 μm: grind and pulverize lactose with a high-speed grinding pulverizer to obtain a micropowder with a particle size of 60 μm to 80 μm; weigh 200 g Mix pidotimod micropowder with 150g lactose micropowder, fill No. 1 capsule, each filling 350mg, make 1000 capsules, and get ready.
Embodiment 3
[0056] Embodiment 3: Preparation of pidotimod inhalation powder aerosol
[0057] Use an ultrafine pulverizer to pulverize pidotimod to obtain an ultrafine dry powder with a particle diameter of 1 μm to 5 μm: grind and pulverize the lactose with a high-speed grinding pulverizer to obtain a micropowder with a particle size of 60 μm to 80 μm; weigh 300 g Domod micro-powder and 100g lactose micro-powder, mix well, fill No. 1 capsule, each capsule is filled with 400mg, make 1000 capsules, and get ready.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com