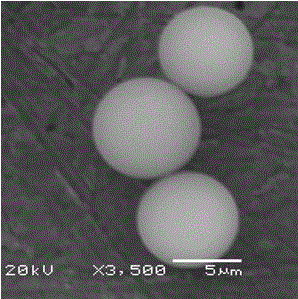
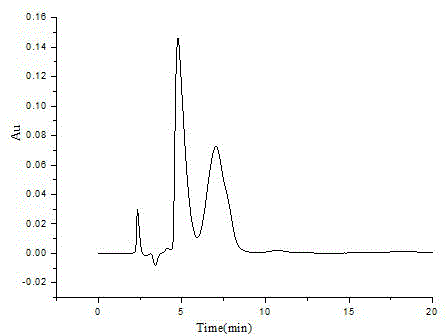
Preparation method of organic-inorganic hybrid cyclodextrin chiral stationary phase
A chiral stationary phase and cyclodextrin technology, which is applied in chemical instruments and methods, and other chemical processes, can solve problems such as limiting the amount of cyclodextrin bonds, complex processes, and harsh reaction conditions, and achieve enhanced chiral separation performance, improving column permeability, and accelerating mass transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 The preparation method of organic-inorganic hybrid cyclodextrin chiral stationary phase, the specific implementation steps are as follows:
[0024] (1) Preparation process of silicon-β-cyclodextrin derivatives
[0025] Put 3g of chlorotriazine-β-cyclodextrin, 2g of aminopropyltriethoxysilane and 1.5g of sodium bicarbonate in a round bottom flask filled with 50mL of water, and magnetically stir in a water bath at 50°C for 30min. After the reaction was completed, 80 mL of ethanol was added, and the silicon-β-cyclodextrin derivative precipitated out, and the precipitate was collected by filtration and dried for later use.
[0026] (2) Preparation of organic-inorganic hybrid cyclodextrin chiral stationary phase by hydrothermal synthesis
[0027] 0.3 g of silyl-β-cyclodextrin derivative prepared in step 1), 1.5 g of silylating agent 1,2-bis(trimethoxysilyl)ethane and 1 g of cetyltrimethylammonium bromide The surfactant was dissolved in 25mL of water, 12mL of etha...
Embodiment 2
[0031] Example 2 The preparation method of organic-inorganic hybrid cyclodextrin chiral stationary phase, the specific implementation steps are as follows:
[0032](1) Preparation process of silicon-β-cyclodextrin derivatives
[0033] Put 4g of chlorotriazine-β-cyclodextrin, 3g of aminopropyltriethoxysilane and 1.5g of sodium bicarbonate into a round bottom flask filled with 50mL of water, and magnetically stir in a water bath at 50°C for 30min. After the reaction was completed, 80 mL of ethanol was added, and the silicon-β-cyclodextrin derivative precipitated out, and the precipitate was collected by filtration and dried for later use.
[0034] (2) Preparation of organic-inorganic hybrid cyclodextrin chiral stationary phase by hydrothermal synthesis
[0035] 0.4 g of the silyl-β-cyclodextrin derivative prepared in step 1), 1.7 g of the silylating agent 1,2-bis(trimethoxysilyl)ethane and 1.2 g of hexadecyltrimethylchloride Ammonium surfactant was dissolved in 25mL of water, ...
Embodiment 3
[0039] Example 3 The preparation method of organic-inorganic hybrid cyclodextrin chiral stationary phase, the specific implementation steps are as follows:
[0040] (1) Preparation process of silicon-β-cyclodextrin derivatives
[0041] Put 5g of chlorotriazine-β-cyclodextrin, 4g of aminopropyltriethoxysilane and 1.5g of sodium bicarbonate into a round bottom flask filled with 50mL of water, and magnetically stir in a water bath at 50°C for 30min. After the reaction was completed, 80 mL of ethanol was added, and the silicon-β-cyclodextrin derivative precipitated out, and the precipitate was collected by filtration and dried for later use.
[0042] (2) Preparation of organic-inorganic hybrid cyclodextrin chiral stationary phase by hydrothermal synthesis
[0043] 0.4 g of the silyl-β-cyclodextrin derivative prepared in step 1), 1.7 g of the silylating agent 1,2-bis(trimethoxysilyl)ethane and 1.2 g of octadecyltrimethylchloride Ammonium surfactant was dissolved in 25mL of water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com