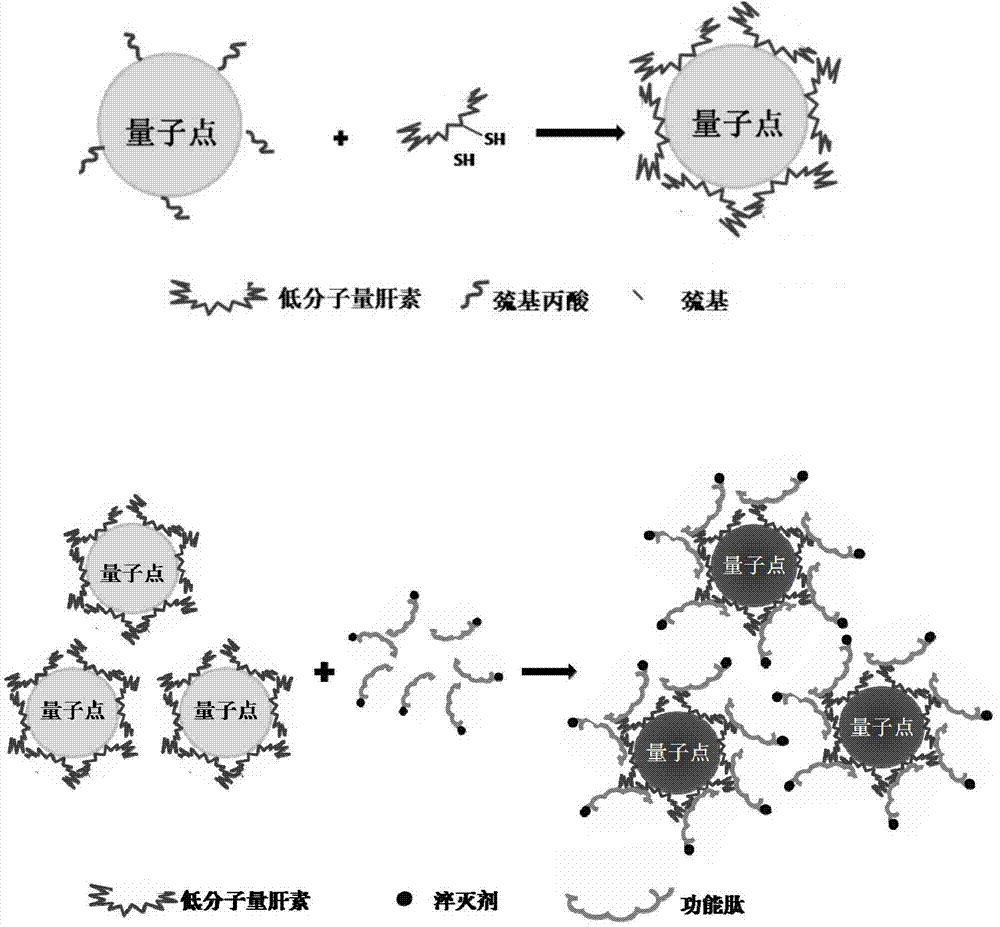
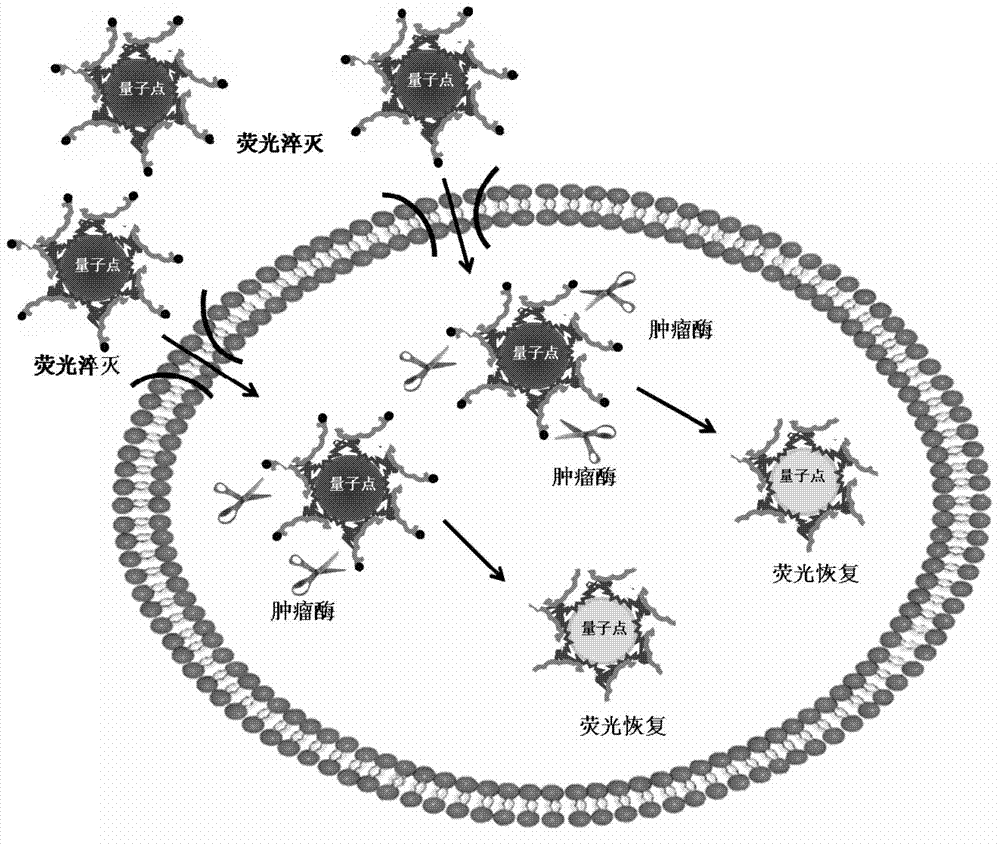
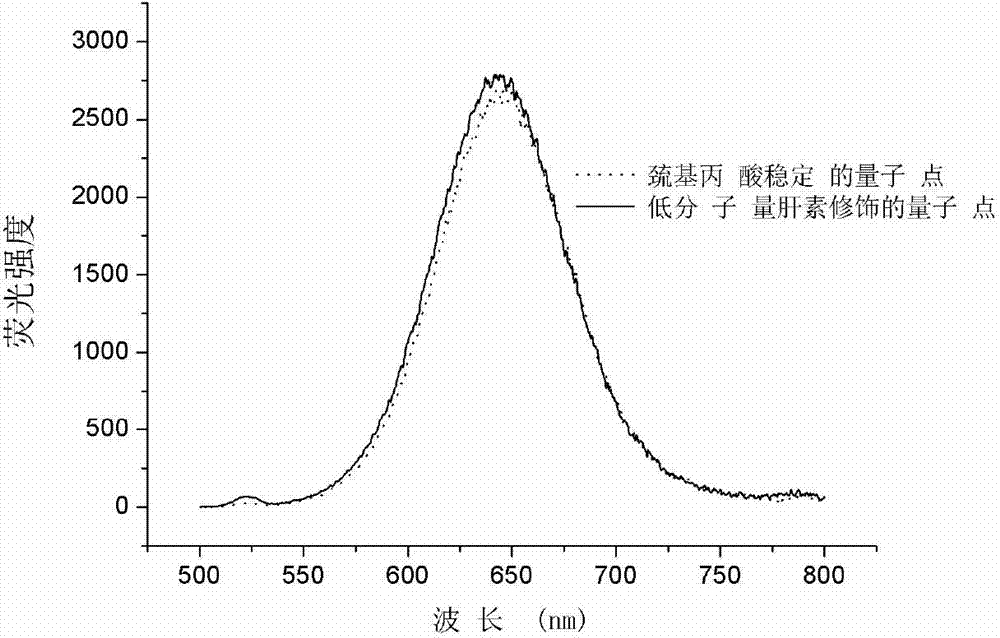
Tumor enzyme activated quantum dot probe, preparation method and application thereof
A tumor enzyme and quantum dot technology, applied in the field of tumor enzyme-activated quantum dot probes and their preparation, can solve the problems of wide emission spectrum and narrow excitation spectrum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Synthesis and purification of embodiment 1 mercaptopropionic acid stable quantum dots
[0064] a Weigh 25.4mg of tellurium powder (purchased from Sinopharm Chemical Reagent Co., Ltd.) and 50mg of sodium borohydride (purchased from Sinopharm Chemical Reagent Co., Ltd.) into a round bottom flask, add 5ml of ultrapure water under nitrogen protection, 80 ℃ oil bath reaction 1-1.5h to purple, that is, sodium hydride telluride precursor solution.
[0065] b Weigh 45.7 mg of cadmium chloride hydrate (purchased from Sinopharm Chemical Reagent Co., Ltd.), add 30 μL of mercaptopropionic acid solution with a purity of 99% (purchased from Alfa Aesar), continue to add 40 ml of ultrapure water, stir until fully dissolved, and adjust pH to 11.9, under the protection of nitrogen, add dropwise one-tenth of the sodium telluride hydride precursor solution prepared in step a, and reflux the reaction at an appropriate time, monitor the reaction result with a fluorescence spectrophotometer (...
Embodiment 2
[0068] Synthesis of Example 2 Thiolated Low Molecular Weight Heparin
[0069] a Dissolve 50mg of low molecular weight heparin (enoxaparin sodium, molecular weight 3500-5500, purchased from Hebei Changshan Biochemical Pharmaceutical Co., Ltd.) in 10ml of citric acid buffer (0.1M, pH3.0), add 21.4mg of periiodine sodium heparin, react at room temperature for 2 hours, and dialyze with a dialysis bag with a molecular weight cut-off of 3500 to obtain oxidized low-molecular-weight heparin.
[0070] b. React the oxidized low-molecular-weight heparin prepared in step a with 11.4 mg of cysteine hydrochloride in a total volume of 20 ml of phosphate buffer (0.1 M, pH 7.0) for 2 h at room temperature.
[0071] c. Slowly add 10ml of newly prepared 0.33M sodium borohydride aqueous solution dropwise to b, react at room temperature for 1 hour, dialyze with a dialysis bag with a molecular weight cut-off of 3500, and store at 4°C for later use.
Embodiment 3
[0072] Example 3 Synthesis of thiolated low molecular weight heparin-modified quantum dots
[0073] a. Add 1 / 5 volume of newly prepared 0.33M sodium borohydride aqueous solution to the stock solution obtained in Example 2, and react at room temperature for 1 h.
[0074] B the mercaptopropionic acid stable quantum dot obtained in embodiment 1 is diluted to OD with PBS 310 =1.0, add different amounts obtained from step a in Example 3, and react in the dark for 12 hours.
[0075] c Purification: Add 3-4 times the volume of isopropanol to b, mix well, centrifuge at 8000g for 20min, discard the supernatant, add 10-20ml of isopropanol, mix well, centrifuge at 8000g for 20min, discard the supernatant, and vacuum dry at 60°C 2h that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com