Preparation method of electrocatalyst of proton exchange membrane fuel cell
A proton exchange membrane and fuel cell technology, applied in battery electrodes, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of strict preparation process requirements, difficult to achieve large-scale, cumbersome preparation process, etc., to achieve controllable Good performance, easy large-scale industrial application, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
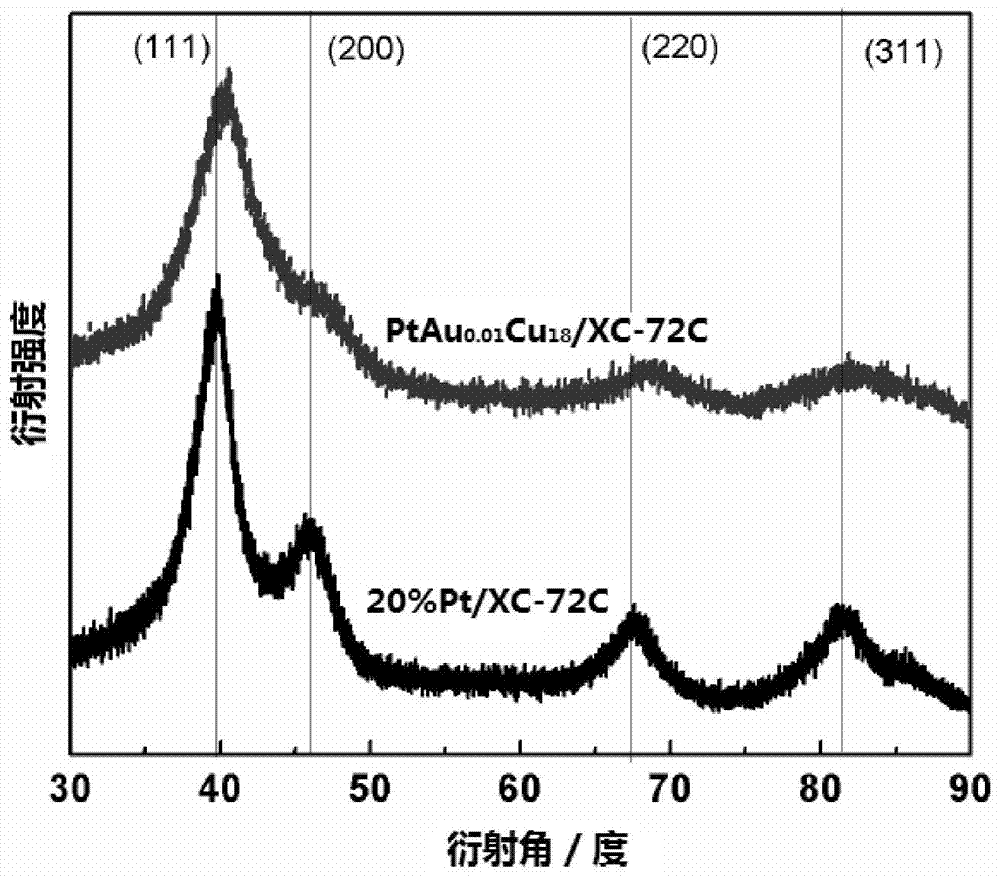
Image
Examples
Embodiment 1
[0065] 1) Under the condition of stirring at room temperature, 223mg Cu(NO 3 ) 2 ·3H 2 O (M: 241.6) is evenly dissolved in 23ml propylene glycol;
[0066] 2) Under the condition of stirring at room temperature, add 7.5ml trisodium citrate (SC) propylene glycol solution to 1), the concentration of the solution is 0.04gSC / ml;
[0067] 3) Pass high-purity Ar into 2 for more than 30 minutes, and the flow rate of Ar is 60ml min -1 ;
[0068] 4) Dissolve 31.5mg sodium borohydride in 3ml deionized water;
[0069] 5) Slowly add the aqueous solution in 4) to 3) at room temperature and high argon atmosphere, and the dropping rate is controlled at 0.15ml s -1 , the reduction reaction starts immediately, and after the reaction is carried out for 2h, Cu nano-seeds stable in colloidal form are obtained, and its concentration is 27.5mM;
[0070] 6) During the reaction in 5), prepare 15mM H 2 PtCl 6 aqueous solution and 10mM HAuCl 4 aqueous solution.
[0071] Pipette 3.3ml of 15mM H...
Embodiment 2
[0088] 1) Under the condition of stirring at room temperature, 230mg Co(CH 3 COO) 2 4H 2 O (M: 249.1) was uniformly dissolved in 92ml of ethylene glycol;
[0089] 2) Under the condition of stirring at room temperature, add 8.6ml EDTA disodium (M: 372.2) ethylene glycol solution to 1), the solution concentration is 0.02g EDTA disodium / ml.
[0090] 3) Pass high-purity N into 2 2 More than 40min, N 2 The flow rate is 100ml min -1 .
[0091] 4) Dissolve 100mg potassium borohydride in 3ml deionized water;
[0092] 5) At room temperature, high N 2 Under the atmosphere, slowly add the aqueous solution in 4) to 3) dropwise, and the dropping rate is controlled at 0.3ml s -1 , the reduction reaction started immediately, and after the reaction was carried out for 3h, the stable Co nanoseeds in the form of colloids were obtained, and the concentration was 8.4mM.
[0093] 6) During the reaction in 5), prepare 5mM as K 2 PtCl 4 aqueous solution and 2mM KAuCl 4 aqueous solution. ...
Embodiment 3
[0102] 1) Under the condition of stirring at room temperature, 0.328g NiCl 2 ·6H 2 O (M: 327.5, 1mmol) was uniformly dissolved in 14.3ml glycerol (70mM);
[0103] 2) Under the condition of stirring at room temperature, add 60ml of CTAC (M:320) glycerol solution to 1), the concentration of this solution is 50mM.
[0104] 3) Pass high-purity He into 2 for more than 40 minutes, and the flow rate of He is 10ml min -1 .
[0105] 4) Slowly add 22 μL of 37% formaldehyde solution to 3) at room temperature and in a high He atmosphere, and the dropping rate is controlled at 0.01ml s -1 , the reduction reaction started immediately, and after the reaction was carried out for 5h, the stable Ni nanoseeds in the form of colloid were obtained, and the concentration was 13.4mM.
[0106] 5) During the reaction in 4), prepare 30mM PtCl 4 aqueous solution and 5mMAuCl 3 aqueous solution.
[0107] 6) Draw 6.7ml, 30mM as PtCl 4 aqueous solution and 268μL, 5mMKAuCl 4 Aqueous solution, after ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com