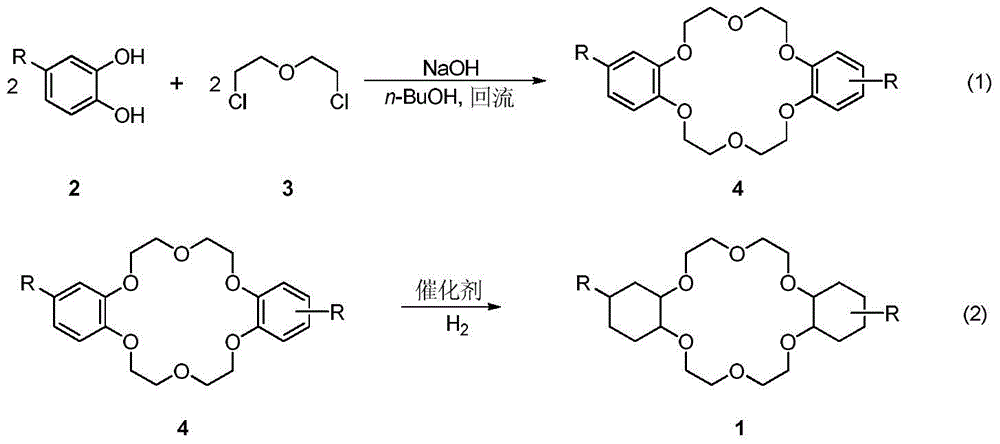
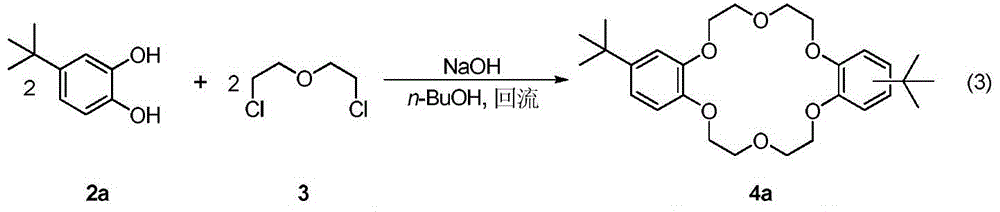
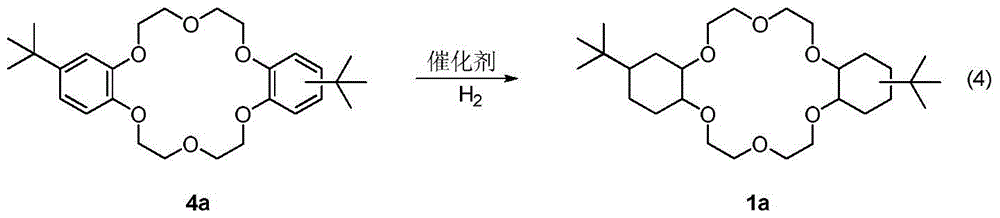
A kind of synthetic method of dicyclohexyl crown ether
A synthetic method, dicyclohexyl technology, which is applied in the field of improved synthetic technology of dicyclohexyl crown ether compounds, can solve the problems of rare synthetic methods, and achieve the effects of low price, simple treatment, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Ni-Ru / γ-Al 2 o 3 Catalyst preparation, weigh γ-Al 2 o 3 (180m 2 / g, 50g) is ground into powder, and the powder particles are larger than 20 mesh. Weigh nickel acetate tetrahydrate (3.1g, 12.5mmol) and ruthenium trichloride trihydrate (3.27g, 12.5mmol), add the above materials into a 250mL round bottom flask, add water (50mL) to soak and stir for 12 hours. in N 2 Under protection, 20 mL of aqueous solution containing sodium borohydride (2.5 g) was dropped into the mixture under stirring, and heated to reflux at 100° C. for 10 hours of reaction. After cooling down to room temperature, the solid was obtained by suction filtration, and the solid was washed twice with 200 mL of water. Then the black solid was heated to 450° C. in a muffle furnace for 5 hours and then cooled to room temperature for use. According to inductively coupled plasma atomic emission spectrometry analysis, the Ni content is 1.5% (mass percentage); the Ru content is 2.5% (mass percentage).
Embodiment 2
[0024]
[0025] in N 2 Add 2-hydroxy-4-(tert-butyl)-phenol 2a (50.8g, 0.31mol), NaOH (12.4g, 0.31mol) and 500mL n-BuOH into a 2L reaction flask under atmosphere, stir and heat to reflux. A solution containing dichloroethyl ether (21.9 g, 0.15 mol) and n-BuOH (50 mL) was added dropwise into the reaction solution within 30 minutes, and the drop was completed within 30 minutes, and then refluxed for 1 hour. The reaction solution was cooled to below 90°C, another portion of NaOH (12.4 g, 0.31 mol) was added, and the temperature was raised to reflux for 30 min. Dichloroethyl ether (21.9 g, 0.15 mol) and n-BuOH (50 mL) were mixed and added dropwise to the reaction liquid, stirred, and refluxed for 15 hours. After the reaction is complete, cool to room temperature and adjust the pH value to 5 with concentrated hydrochloric acid. Evaporate the solvent n-BuOH, add an appropriate amount of water as the volume of the mixture decreases, and continue heating until the temperature of t...
Embodiment 3
[0027]
[0028] 4,4'(5')-bis(tert-butyl)diphenyl-18-crown-6 ether 4a (10g, 20.5mmol), catalyst Ni-Ru / γ-Al 2 o 3 (10.0g, the molar ratio of Ni to Ru is 1:1), tetrahydrofuran (100mL) was added into a 250mL pressure reactor, and sealed. h 2 Filled with H after replacement 3 times 2 To 8Mpa pressure, the oil bath is heated to 180 ℃ and stirred. React for 2 hours, put the reaction kettle into cold water and drop to room temperature, the pressure drops to 5MPa, add H 2 Continue to react overnight to 8MPa. After reacting for 24 hours, the reaction kettle was put into cold water and dropped to room temperature (pressure dropped to 2Mpa), supplemented with H 2 Continue to react to 8MPa, at this time the reaction speed is obviously slowed down, and the pressure drop is not obvious. After a total reaction of 30 hours, the reaction kettle was put into cold water and the pressure was lowered to 6 MPa at room temperature to stop the reaction. The reaction mixture was filtered to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com