A kind of recombinant active peptide and its synchronous preparation method
A technology of active peptides and peptide segments, applied in the field of genetic engineering, can solve the problems of lack of high in vivo activity and low cost, and achieve the effects of improving vascular endothelial cell function, increasing production rate, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
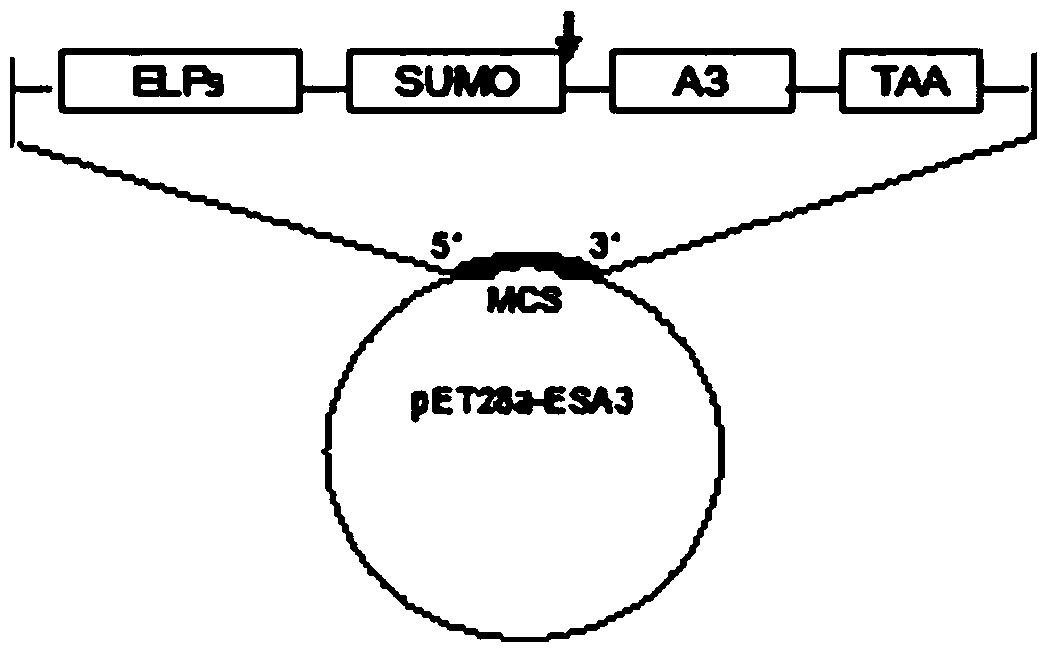
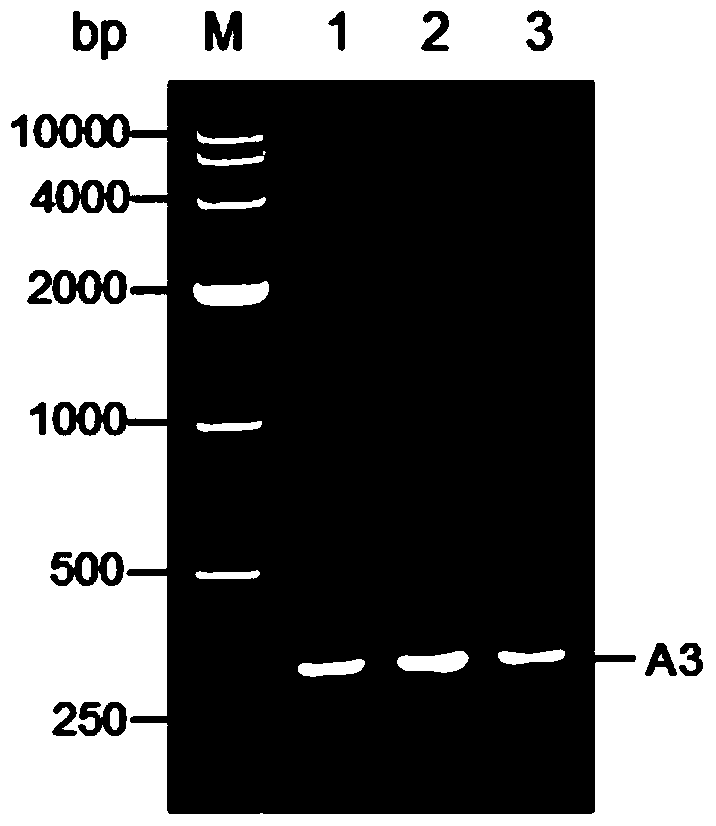
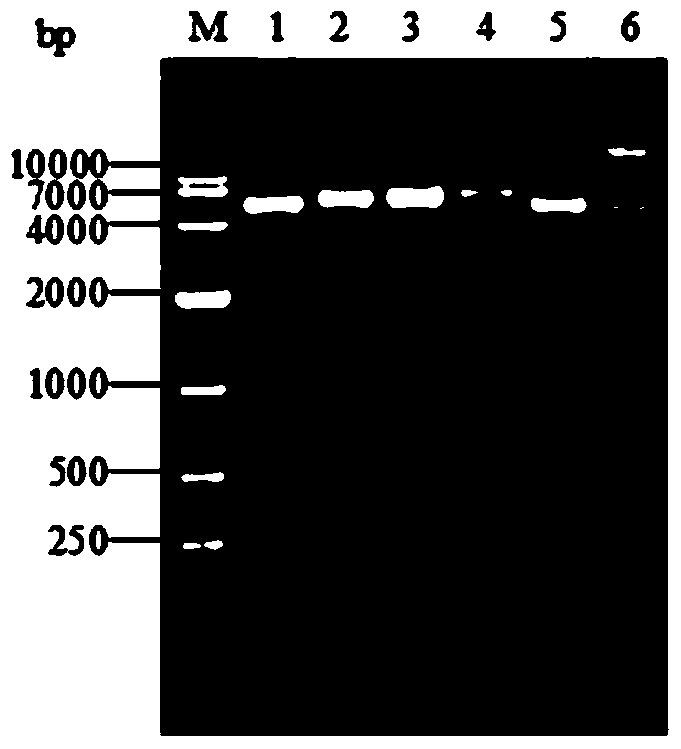
[0041] Such as Figure 1 to Figure 7 as shown, figure 1 Schematic diagram of the recombinant plasmid constructed for the present invention; figure 2 It is a schematic diagram of the PCR identification of the recombinant plasmid of the present invention. Lane M is the DL10000 DNA standard sample, and lanes 1, 2, and 3 are all the same PCR product (using the recombinant plasmid pET28a-ESA3 as a template, primers 5'-TTCCTGGAACCGGA-3' and 5'- CCAAGCCACACGA AAC-3' is the upstream and downstream primers); image 3 It is a schematic diagram of the double enzyme digestion identification of the recombinant plasmid of the present invention, M is DNA Marker, and lanes 1-5 are Nco I / Bam HI, Bam HI / Hind III, Hind III / Xho I, Bam HI / Xho I, Nco I respectively / Xho I double digestion product, lane 6 is the recombinant plasmid; Figure 4 SDS-PAGE is a schematic diagram of the identification of recombinant protein inclusion body expression. Lane M is the protein standard sample, and lanes 1,...
Embodiment 2
[0079] The difference between Example 2 and Example 1 is that the engineering bacteria pET28a-ESA3 / E.coli BL21(DE3) was constructed according to the steps (1) and (2) in Example 1. Put a single colony of engineering bacteria in 20ml of LB medium containing 50μg / ml Kana, culture overnight at 37°C, 200rpm shaker, and then transfer the overnight culture solution into TB containing 50μg / ml Kana at an inoculum size of 2%. In the culture medium, 37°C, 200rpm, shake the flask to cultivate until the OD value of the bacteria reaches 0.6-0.8, add IPTG to the final concentration of 0.02mM, induce culture at 20°C for 20h, and sample for SDS-PAGE analysis.
[0080] Such as Figure 4 It is known that after the engineered bacteria were induced and cultured for 20 hours under the condition of low temperature and low inducer concentration, a protein band with a molecular weight of about 45 kDa appeared relative to the pre-induction sample; after the cells were ultrasonically disrupted, the sol...
Embodiment 3
[0082] The difference between Example 3 and Example 1 lies in the preparation of the recombinant small peptide mixture and its activity identification. Take an appropriate amount of prepared ELPs-SUMO-A3, add SUMO protease according to the addition amount of 3%, and enzymatically hydrolyze at 30°C for 5h. After the enzymolysis, add sodium chloride with a final concentration of 1.5mol / l to the enzymolysis solution, bathe in water at 30°C for 10 minutes, centrifuge at 8000r / min for 10 minutes, and keep the supernatant, which is the recombinant active peptide. Simulate the natural physiological digestion process of the human body in vitro, adjust the pH of the 2mg / ml recombinant active peptide solution to 2.0 with hydrochloric acid, add 2% pepsin, hydrolyze it at 37°C for 4 hours, stop the reaction in a boiling water bath, adjust the pH to 7.0, and take part of the hydrolyzed solution Determination of peptide activity. Add 2% trypsin (or a mixture of trypsin and chymotrypsin) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com