Method for constructing LQT disease model and application of LQT disease model in drug screening
A technology of disease model and culture, applied in the field of constructing LQT disease model, can solve the problem that LQTS disease model needs to be further developed, and achieve the effect of low cancer risk, high safety and high reprogramming efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Retroviral reprogramming
[0050] (1) Donor cells LQTL1 and LQTL2 were cultured with urine cell expansion medium (CIB, Cat#Iso-1030). The day before virus infection, urine epithelial cells were divided into 1 well of 12-well plate according to 20,000 cells.
[0051] (2) Transfect pMXs virus plasmid into 293T cells by PEI transfection method, that is, use 293T cells to package virus pMXs-Oct4(O), pMXs-Sox2(S), pMXs-Klf4(K), pMXs-c-Myc respectively (M) and pMXs-eGFP, the retrovirus encoding pMXs-Sox2 / Klf4 / Oct4 / c-Myc was obtained, and the virus supernatant was filtered with a 0.45 μm filter membrane to obtain the infection solution.
[0052] (3) Add 8 μg / ml polybrene to the infection solution, and use the virus combination OSKM to infect the cells LQTL1 and LQTL2 obtained in step (1) twice, and eGFP-infected cells as a control for infection efficiency, after infection The cells were further cultured with human induced pluripotent stem cell (hiPSC) reprogrammin...
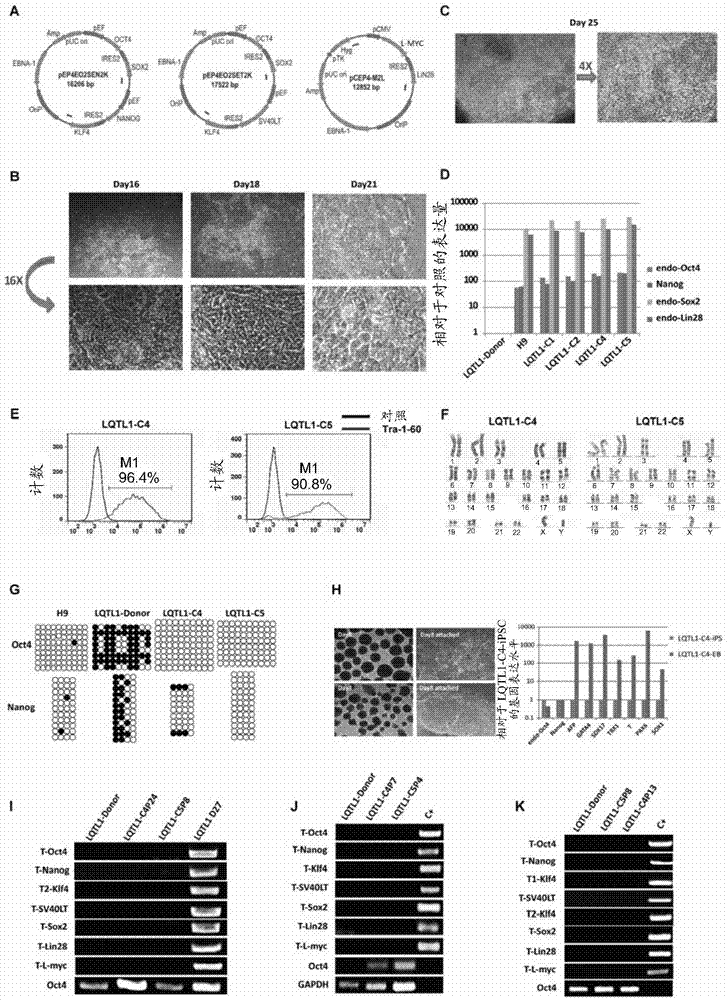
Embodiment 2
[0057] Example 2: Non-integrated iPSC reprogramming
[0058] 1) The donor cell LQTH1 was cultured with urine cell expansion medium (CIB, Cat#Iso-1030).
[0059] 2) Digest the donor cell LQTH1 into single cells with trypsin before transfection, stop the digestion with urine cell expansion medium (CIB, Cat#Iso-1030), centrifuge at 200g for 3mins, discard the supernatant, and use the culture medium The cells were resuspended, counted with trypan blue, and 1 million cells were taken for nucleofection.
[0060] 3) Combining Episome plasmids (purchased from Addgene, where the plasmid pCEP4-M2L uses CIB to replace c-Myc with L- Myc plasmids) were transferred into 1 million donor cells in total, and the structure diagram of the Episome plasmid combination is shown in figure 2 a.
[0061] 4) Spread the transfected cells into 2 ECM-coated T25 flasks, culture them with human induced pluripotent stem cell (hiPSC) reprogramming medium (CIB, Cat#Rep-1101), at 37°C, 5% CO 2 cultured und...
Embodiment 3
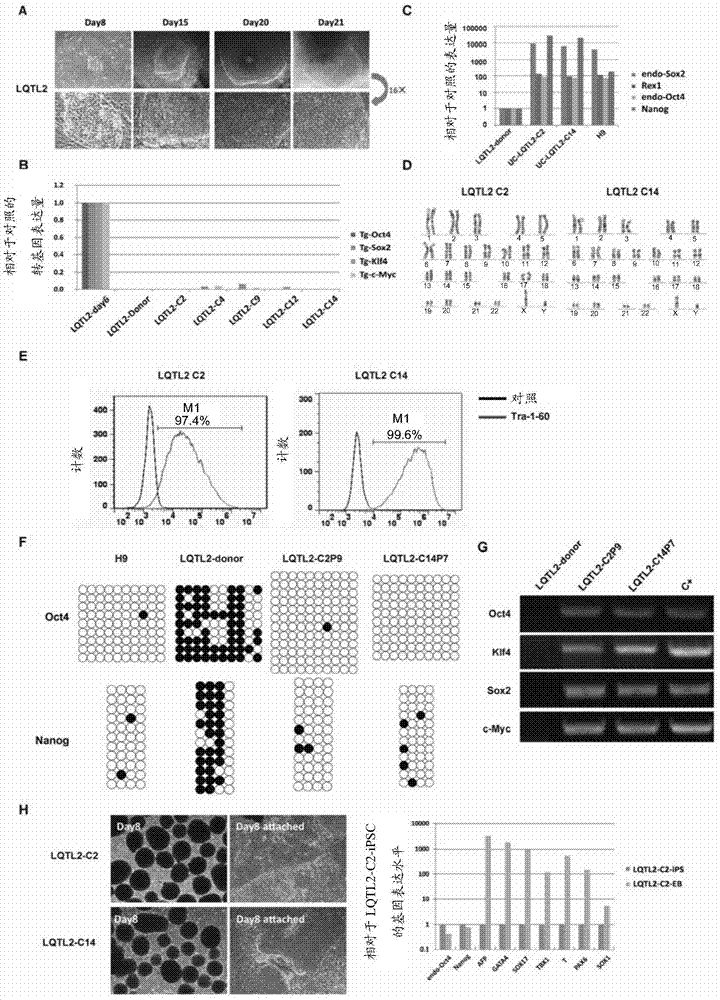
[0065] Example 3: iPSC identification
[0066] (1) Karyotype analysis
[0067] 1) The induced pluripotent stem cells obtained in Example 2 were treated with colchicine 3 hours before the termination of the culture, and the cells were collected by adding trypsin after the termination of the culture.
[0068] 2) Treat with 0.075M hypotonic solution (0.075M hypotonic solution KCl solution), then add fixative solution (glacial acetic acid:methanol=1:3) to fix the cells, and repeat the fixation 1-2 times.
[0069] 3) Depending on the amount of cells, add an appropriate amount of fixative to make a cell suspension, absorb the cell suspension and drop it on a glass slide, and put the slide in an oven to bake.
[0070] 4) Digest slides in trypsin with a pH of 7.0-7.2, and then wash them in NaCl solution at 37°C.
[0071] 5) Stain the slides with Giemsa staining solution, dry them and read them with OLYMPUS, BX41 / BX2.
[0072] (2) Methylation analysis
[0073] In this example, conv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com