Preparation method of pharmaceutical composition for treating digestive system disease
A technology for diseases of the digestive system and composition, applied in the field of medicine, can solve the problems of complex production process, high production cost, small production volume and the like, and achieve the effects of simple preparation method, good stability effect and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
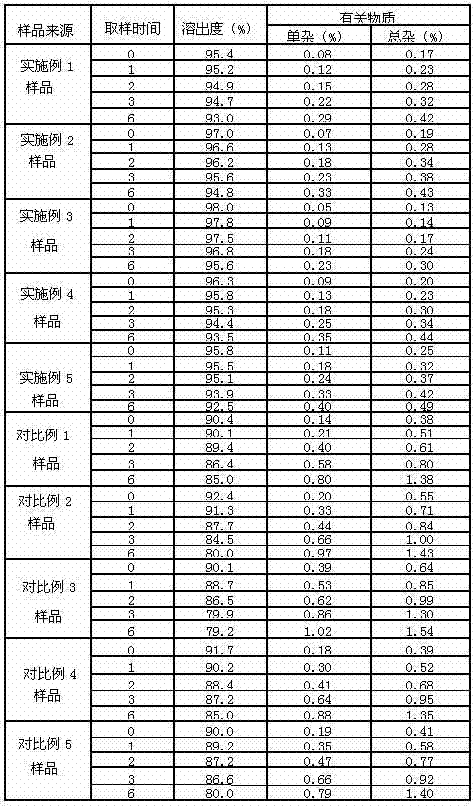
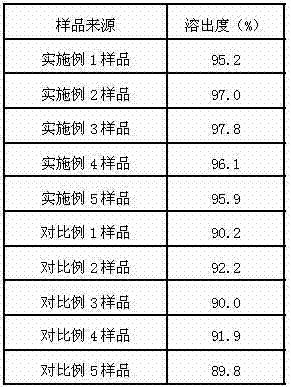
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of lansoprazole enteric-coated tablet
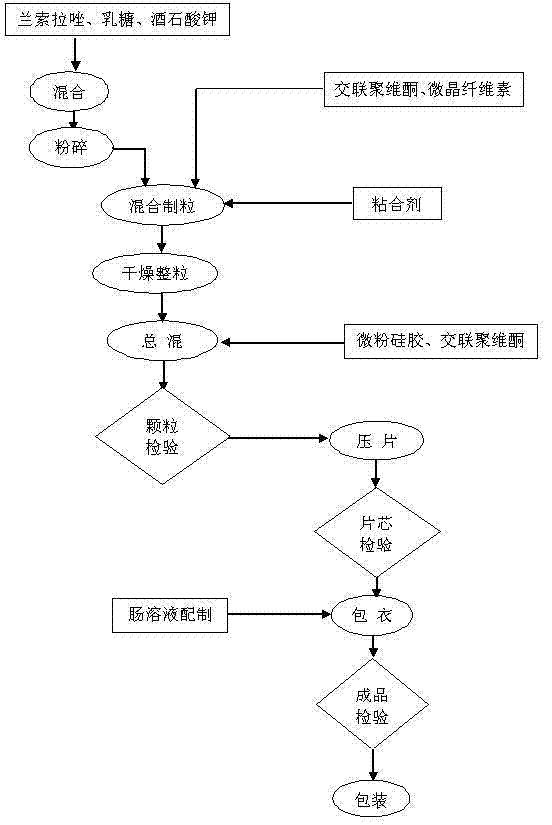
[0050] (1) Premixing and crushing: 10 parts by weight of lansoprazole, 49 parts by weight of lactose, and 8.5 parts by weight of potassium sodium tartrate were mixed with a mixer for 10 minutes at a speed of 15 Hz, and pulverized with a pulverizer after mixing 80 mesh screen, mill speed 30Hz;
[0051] (2) Prepare adhesive: add 1.0 parts by weight of povidone K30 to 20 parts by weight of adhesive solvent 95% ethanol and stir to dissolve, add 0.6 parts by weight of polysorbate 80, and stir evenly;
[0052] (3) Mixing and granulation: Put the mixed and pulverized raw and auxiliary materials in step (1), 15 parts by weight of microcrystalline cellulose, and 6 parts by weight of internally added disintegrant crospovidone in a wet mixing granulator In the process, dry mix for 10 minutes, add the prepared binder, stir and cut at high speed for 150 seconds to obtain a soft material, and use a nylon mesh to c...
Embodiment 2
[0059] Embodiment 2: the preparation of lansoprazole enteric-coated tablet
[0060] (1) Premixing and pulverization: 14 parts by weight of lansoprazole, 52 parts by weight of lactose, and 9.8 parts by weight of potassium sodium tartrate were mixed with a mixer for 10 minutes at a speed of 15 Hz, and pulverized with a pulverizer after mixing 80 mesh screen, mill speed 30Hz;
[0061] (2) Prepare adhesive: add 1.5 parts by weight of povidone K30 to 22 parts by weight of adhesive solvent 95% ethanol and stir to dissolve, add 0.7 parts by weight of polysorbate 80, and stir evenly;
[0062] (3) Mixing and granulation: Put the mixed and pulverized raw and auxiliary materials in step (1), 17 parts by weight of microcrystalline cellulose, and 7.5 parts by weight of internally added disintegrant crospovidone in a wet mixing granulator In the middle, dry mix for 10 minutes, add the prepared binder, stir and cut at high speed for 150 seconds to obtain a soft material, and use a nylon mes...
Embodiment 3
[0069] Embodiment 3: the preparation of lansoprazole enteric-coated tablet
[0070] (1) Premixing and crushing: 15.7 parts by weight of lansoprazole, 54.5 parts by weight of lactose, and 10.5 parts by weight of potassium sodium tartrate were mixed with a mixer for 10 minutes at a speed of 15 Hz, and pulverized with a pulverizer after mixing 80 mesh screen, mill speed 30Hz;
[0071] (2) Prepare adhesive: add 1.9 parts by weight of povidone K30 to 24.1 parts by weight of adhesive solvent 95% ethanol and stir to dissolve, add 0.8 parts by weight of polysorbate 80, and stir evenly;
[0072] (3) Mixing and granulation: Put the mixed and crushed raw and auxiliary materials in step (1), 20.0 parts by weight of microcrystalline cellulose, and 8.3 parts by weight of internally added disintegrant crospovidone in a wet mixing granulator In the process, dry mix for 10 minutes, add the prepared binder, stir and cut at high speed for 150 seconds to obtain a soft material, and use a nylon m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com