Method for preparing beta-phenethyl alcohol
A technology of phenylethyl alcohol and alcohol ester, which is applied in the field of preparation of β-phenylethyl alcohol, can solve problems such as tower blockage, difficulty in separation of auxiliary base products, etc., and achieve high overall yield, overcome difficulty in recycling, and long catalyst life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
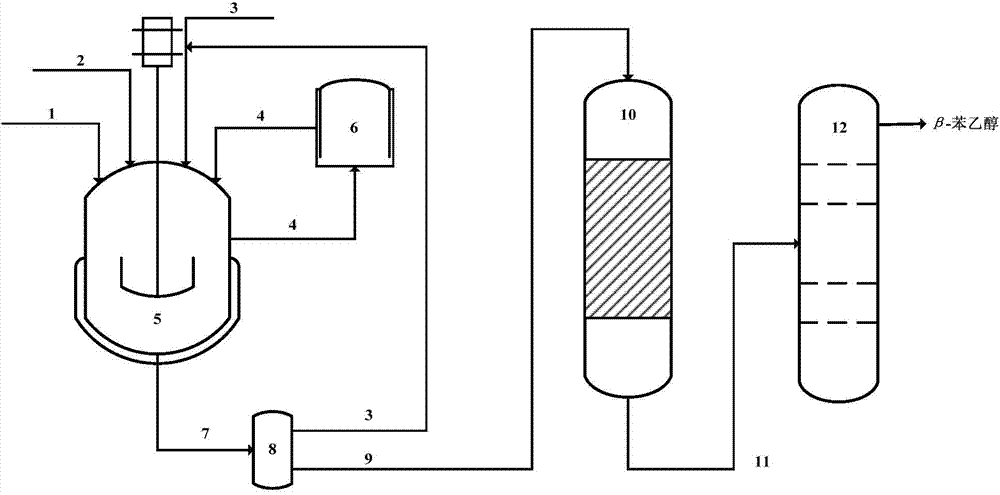
[0049] Add 5.05 g of 15 wt% AlCl to the cycloaddition reactor 3 - Samarium Chloride / C 60 (OH) 26 (AlCl 3 : samarium chloride=45:1), 10g acetic acid-3-butene-1-ol ester and 1000g water, feed 1,3-butadiene into the cycloaddition reactor with the rate of 5L / h and react , the reaction temperature is 25°C, the pressure is normal pressure, and the reaction time is 3h.
[0050] Add 1000 g of 10% LiOH aqueous solution to the reaction liquid, and stir for 10 min for hydrolysis. The reaction results showed that the conversion rate of acetic acid-3-buten-1-ol ester was 97.68%, and the yield of 3-cyclohexene ethanol was 91.47%.
[0051] The 3-cyclohexene ethanol reaction solution obtained by hydrolysis enters the polysulfone membrane module for pervaporation and desolventization treatment. The membrane permeation rate J of the selected membrane module is 0.62Kg m -2 h -1 , the separation coefficient α is 22, the operating temperature is 28°C, and the operating pressure is 1.5Mpa. T...
Embodiment 2
[0055] Add 8.08 g of 22% AlCl to the cycloaddition reactor 3 -Cerium chloride / C 60 (OH) 32 (AlCl 3 : cerium chloride=65:1), 10g acetate-3-butene-1-alcohol ester and 1000g water, feed 1,3-butadiene into cycloaddition reactor with the rate of 8L / h and react , the reaction temperature is 10°C, the pressure is normal pressure, and the reaction time is 2.5h.
[0056] Add 1000 g of 30% NaOH aqueous solution to the reaction liquid, and stir for 10 min for hydrolysis. The reaction results showed that the conversion rate of 3-buten-1-ol acetate was 98.35%, and the yield of 3-cyclohexene ethanol was 93.58%.
[0057] The 3-cyclohexene ethanol reaction solution obtained by hydrolysis enters the poly-cellulose acetate membrane module for pervaporation and desolventization treatment, and the membrane permeation rate J of the selected membrane module is 0.78Kg m -2 h -1 , the separation coefficient α is 35, the operating temperature is 35°C, and the operating pressure is 2.0Mpa. The r...
Embodiment 3
[0061] Add 6.57 g of 35% AlCl to the cycloaddition reactor 3 -Thulium chloride / C 60 (OH) 30 (AlCl 3 : thulium chloride=55:1), 10g acetate-3-butene-1-ol ester and 1000g water, feed 1,3-butadiene into cycloaddition reactor with the rate of 6L / h and react , the reaction temperature is -5°C, the pressure is normal pressure, and the reaction time is 2h.
[0062] Add 800 g of 15% LiOH aqueous solution to the reaction liquid, and stir for 10 min for hydrolysis. The reaction results showed that the conversion rate of 3-buten-1-ol acetate was 99.01%, and the yield of 3-cyclohexene ethanol was 96.16%.
[0063] The 3-cyclohexene ethanol reaction solution obtained by hydrolysis enters the poly-cellulose acetate membrane module for pervaporation and desolventization treatment, and the membrane permeation rate J of the selected membrane module is 0.70Kg m -2 h -1 , the separation coefficient α is 30, the operating temperature is 30°C, and the operating pressure is 1.8Mpa. The reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com