Inhalation pharmaceutical composition used for treatment of chronic obstructive pulmonary disease (COPD) and asthma and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, heterocyclic compound active ingredients, etc., can solve the problems of unsatisfactory existing treatments for asthma and COPD, difficulty in obtaining clinical curative effect, etc., and achieve the goal of reducing adverse effects Incidence, Avoidance of Respiratory Irritation, Effects of Ease of Manipulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A pharmaceutical composition in dry powder form, its prescription is as shown in the following table:
[0031] Raw materials
Dosage (mg)
Formoterol Fumarate Dihydrate (A)
40
Budesonide (B)
800
Tiotropium bromide monohydrate (C)
100
lactose monohydrate
99060
production
10000 capsules
[0032] Preparation:
[0033] 1) (A), (C) and 9 times the amount of carrier lactose are mixed in equal increments and uniform, and then jet milled to less than 10 μm to make the particle part.
[0034] 2) (B) is pulverized to a particle size of less than 10 μm by air flow.
[0035]3) An appropriate amount of carrier lactose is ball milled to a particle size below 212 μm to prepare a fine particle carrier.
[0036] 4) Add the micropowder obtained in 1) and 2) to the fine granules obtained in 3) at one time, measure the content after mixing, and fill capsules according to the content.
Embodiment 2-129
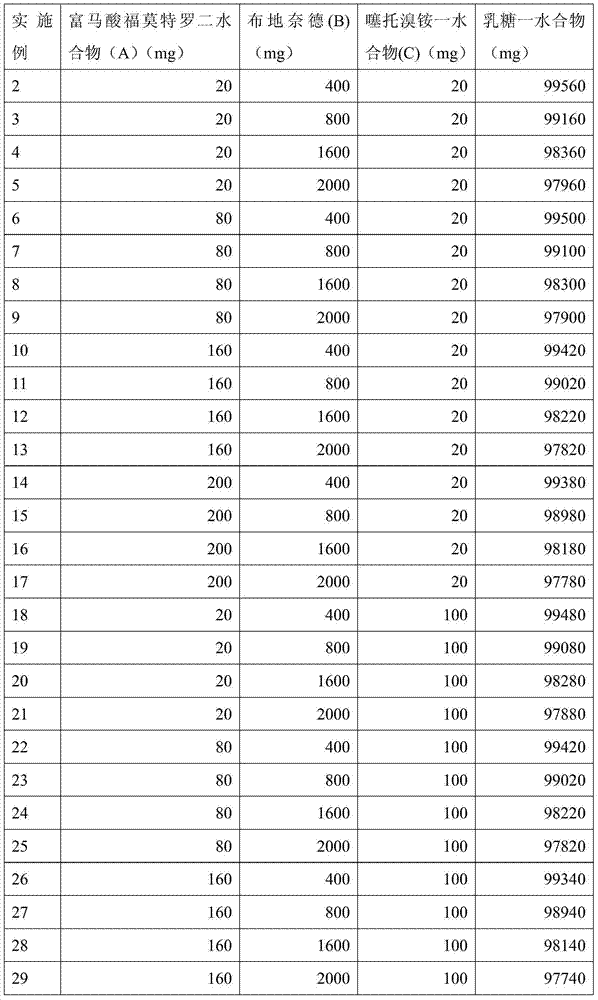
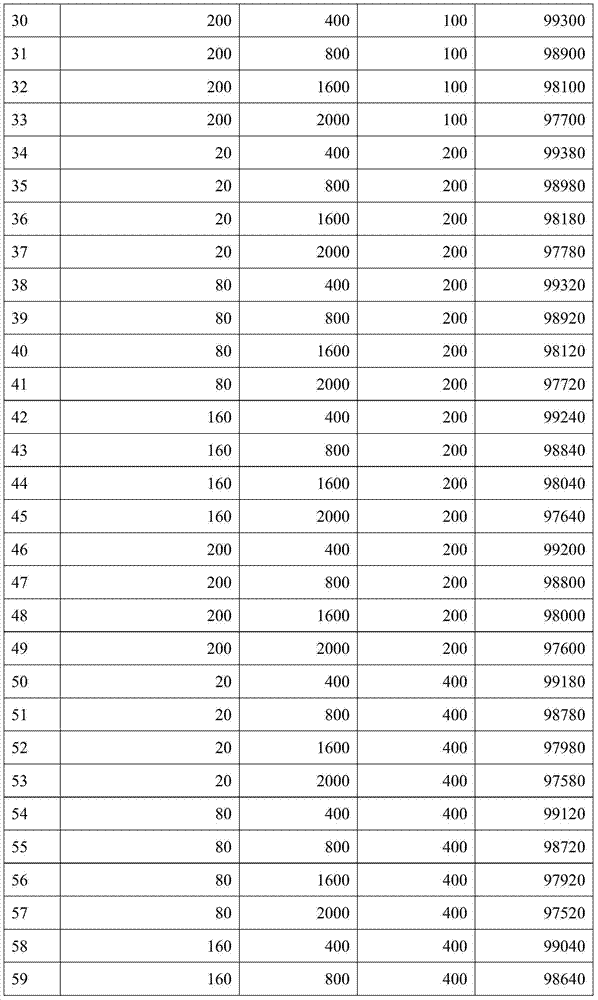
[0038] It is basically the same as implementation 1, but the contained components of each corresponding embodiment use the amount of the composition shown in the table below:
[0039]
[0040]
[0041]
[0042]
[0043]
Embodiment 130
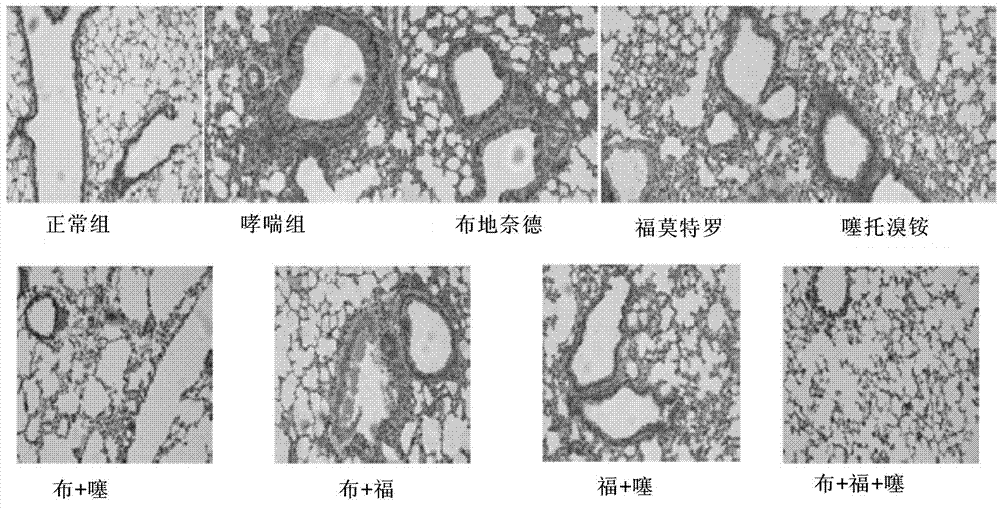
[0044] Example 130 Study on the therapeutic effect of the composition on mice with asthma
[0045] 1. Experimental animals and groups:
[0046] SPF grade BALB / c mice, female, 6-7 weeks old, weighing 18-20 grams. Ovalbumin-depleted (egg-free) special feed and water were given ad libitum. Randomly divided into the following groups:
[0047] Normal control group (n=8): normal saline sensitization, challenge and intervention; hereinafter referred to as the normal group;
[0048] Asthma control group (n=8): OVA sensitization, challenge, normal saline intervention; hereinafter referred to as the asthma group;
[0049] Budesonide group (n=8): OVA sensitization, challenge, budesonide saline solution (200ug / ml) intervention;
[0050] Formoterol group (n=8): OVA sensitization, challenge, formoterol fumarate dihydrate saline solution (10ug / ml) intervention;
[0051] Tiotropium bromide group (n=8): OVA sensitization, challenge, tiotropium bromide monohydrate saline solution (50ug / ml)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com