Method for producing methacrolein and methacrylic acid
A technology for methacrylic acid and methacrolein, applied in chemical instruments and methods, carboxylate preparation, organic chemical methods, etc., can solve the problems of reduced conversion rate of raw materials, decreased catalyst activity, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
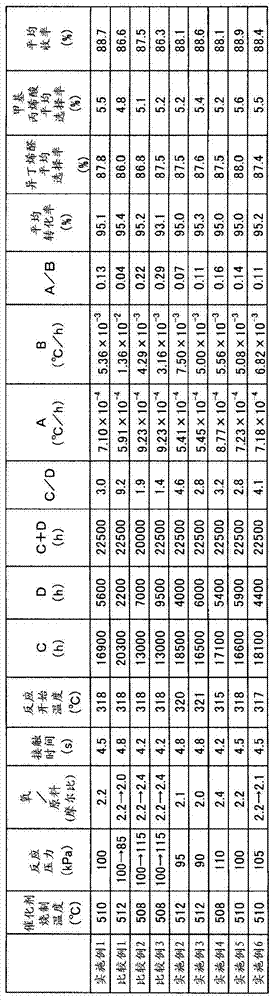
Embodiment 1
[0072] (manufacture of catalyst)
[0073] 3000 parts of ammonium paramolybdate was dissolved in 6000 parts of pure water. Next, 74.4 parts of ammonium paratungstate, 138.0 parts of cesium nitrate, 164.4 parts of antimony trioxide, and 198.0 parts of bismuth trioxide were added, stirring this solution, and it heated to 50 degreeC (A liquid). In addition, 1258.8 parts of iron nitrate, 453.0 parts of nickel nitrate, 2719.8 parts of cobalt nitrate, 187.8 parts of lead nitrate and 33.6 parts of 85% phosphoric acid were successively added to 6000 parts of pure water to dissolve, and heated to 30° C. (B liquid). Under stirring, add liquid B to liquid A to obtain an aqueous slurry, which is aged at 90° C. for 2 hours. Then, after heating up to 103 degreeC and concentrating for 1 hour, dry powder was obtained using the spray dryer. The obtained dry powder was fired once at 300° C. for 1 hour, and then fired twice at 510° C. for 3 hours to obtain a fired catalyst powder.
[0074] Aft...
Embodiment 2
[0109] (manufacture of methacrolein and methacrylic acid)
[0110] The reaction tube used in Example 1 was filled with 2150 g of the catalyst produced in Comparative Example 1. Next, a reaction gas composed of 5% by volume of isobutylene (reaction raw material), 10.5% by volume of oxygen, 10% by volume of water vapor, and 74.5% by volume of nitrogen was passed through the catalyst layer at a reaction pressure of 95 kPaG and a contact time of 4.8 seconds. The gas-phase catalytic oxidation of isobutylene was carried out in the same manner as in Example 1 so that the target conversion rate of the raw material was 95%. It should be noted that the reaction start temperature for gas-phase catalytic oxidation of isobutylene was 320°C.
[0111] The time C (hour) from the start of the reaction until the reaction temperature reached TA was 18500 hours, the time D (hour) from the time the reaction temperature reached TA to the end of the reaction was 4000 hours, and C / D was 4.6. It sho...
Embodiment 3
[0113] (manufacture of methacrolein and methacrylic acid)
[0114] The reaction tube used in Example 1 was filled with 2150 g of the catalyst produced in Comparative Example 1. Next, a reaction gas composed of 5% by volume of isobutylene (reaction raw material), 10% by volume of oxygen, 10% by volume of water vapor, and 75% by volume of nitrogen was passed through the catalyst layer at a reaction pressure of 90 kPaG and a contact time of 4.8 seconds. The gas-phase catalytic oxidation of isobutylene was carried out in the same manner as in Example 1 so that the target conversion rate of the raw material was 95%.
[0115] The reaction start temperature for gas-phase catalytic oxidation of isobutylene was 321°C. The time C (hour) until the reaction temperature reached TA was 16,500 hours, the time D (hour) from when the reaction temperature reached TA to the end of the reaction was 6,000 hours, and C / D was 2.8. It should be noted that the time of the whole reaction is 22500 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com