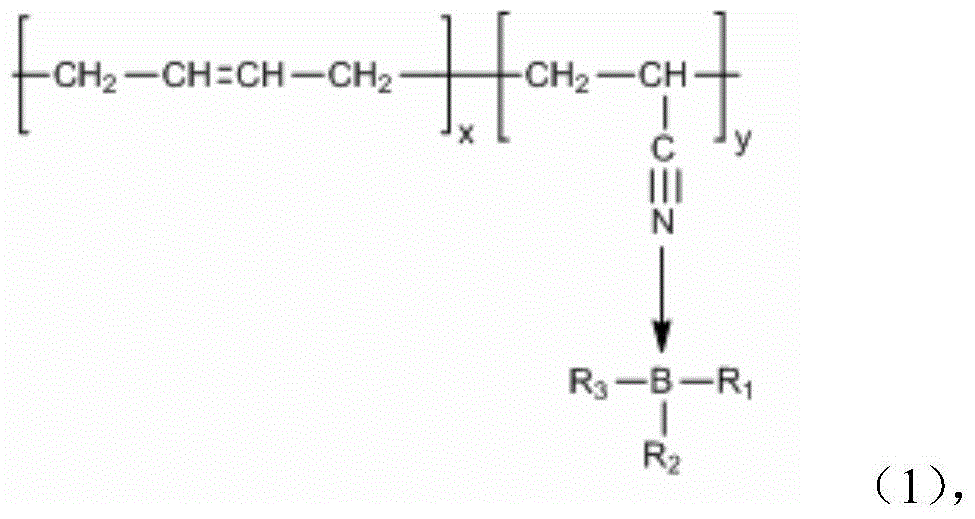
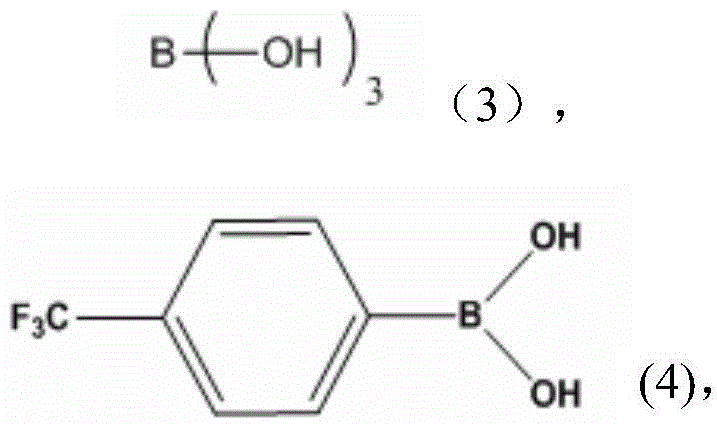
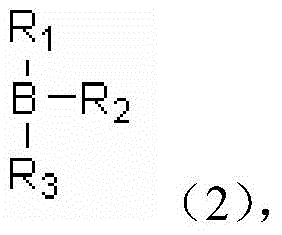A kind of coordination branched nitrile rubber polymer and preparation method thereof
A technology of rubber polymer and branched nitrile, which is applied in the field of coordination branched nitrile rubber polymer and its preparation, can solve the problems of low lithium ion migration number, limited application, electrode polarization, etc. High ionic conductivity and ion migration number, the effect that meets the requirements of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 2.89g of polyethylene glycol monomethyl ether with a molecular weight of 750 and 0.13g of trimethyl borate into the butanone solvent, connect the water separator and the reflux of the condenser tube, place a little aluminum chloride in advance on the water separator, and the mixture Heating to 60 DEG C under stirring state and nitrogen atmosphere, after 3 hours, the temperature is raised to 80 DEG C, after continuing to react for 6 hours, the methyl ethyl ketone solution that will be dissolved with 0.20g nitrile rubber is heated in the reaction device, Keep the temperature constant and continue to stir for 3 hours, then cool down, then concentrate and dry to obtain a coordination branched nitrile rubber polymer.
Embodiment 2
[0032] Pour 50ml of benzene into a three-necked flask containing 2.00g of catechol and 1.13g of boric acid, connect the water separator and condenser tube, and heat to 110°C under the protection of nitrogen. After 5 hours of reaction, the dissolved 14.32 The benzene solution of polyethylene glycol monomethyl ether of 1 g was added in the reaction flask, continued to maintain the same temperature for 8 hours, then filtered, concentrated, and dried to obtain borate, and 1.08 g of the borate obtained was added to the dissolved 0.20 g of nitrile rubber in tetrahydrofuran solution, heated to 60° C., stirred for 2 hours, cooled, then concentrated and dried to obtain a coordination branched nitrile rubber polymer.
Embodiment 3
[0034] Pour 70ml of tetrahydrofuran (THF) solution into a three-necked flask containing 4.00g of catechol and 3.78g of trimethyl borate, stir and heat to 60°C, and after 3 hours of reaction, raise the temperature to 80°C to continue the reaction for one Hours later, add 20.00g of PEG550 dissolved THF solution into the reaction flask and continue to stir for 5 hours. After concentration, add benzene, filter, concentrate, and dry to obtain borate. 0.48g of nitrile rubber in tetrahydrofuran solution, heated to 70°C, stirred for 3 hours, cooled, then concentrated and dried to obtain coordination branched nitrile rubber polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com