Chlorogenic acid acylate capable of improving bioavailability of chlorogenic acid and application thereof
A technology of chlorogenic acid acylate and chlorogenic acid, which is applied in the field of biomedicine, can solve the problems of low bioavailability, unstable polyphenolic compounds, and difficulty in fully exerting the drug effect, so as to improve blood drug concentration, Increased bioavailability, enhanced inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
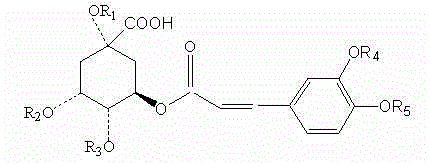
[0025] A chlorogenic acid acylate that improves the bioavailability of chlorogenic acid, characterized in that it has the following structural formula:
[0026]
[0027] where: R 1 , R 2 , R 3 , R 4 , R 5 for RCO + functional group or H + ion, RCO + Functional groups in R 1 , R 2 , R 3 , R 4 , R 5 any one or more of these positions.
[0028] The RCO + R in the functional group is an alkyl group containing 1-6 carbon atoms.
[0029] The chlorogenic acid acylate with such a structure can improve bioavailability and enhance drug efficacy.
[0030] The medicine is an oral preparation prepared by adding chlorogenic acid acylate as an active ingredient and adding one or more pharmaceutically acceptable excipients.
[0031] Wherein, the oral preparations are tablets, capsules, granules, powders, pills, and oral liquids.
[0032] The unit preparation of the oral preparation contains 1-1000 mg of chlorogenic acid acylate, and in the optimized scheme, the unit prepara...
Embodiment 2
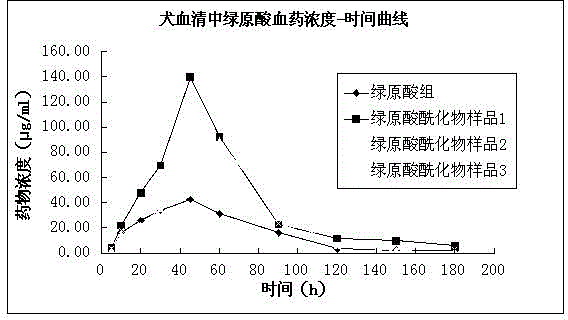
[0038] (1) Chlorogenic acid, commercially available; chlorogenic acid acylate: R 1 , R 2 and R 3 for H + ions, where R 4 , R 5 for C 2 h 3 o + , marked as sample 1; R 1 , R 2 and R 3 for C 3 h 5 o + , where R 4 , R 5 for H + ions, labeled as sample 2; R 1 , R 4 and R 5 for H + , where R 2 , R 3 for C 7 h 5 o + ions, labeled as sample 3.
[0039] The preparation method of sample 1 can be: 100ml DMF electromagnetic stirring, add 50g chlorogenic acid, keep the temperature at 10°C, add 0.1ml pyridine dropwise, then add 10ml acetic anhydride to react for 24 hours, vacuum dry at 80°C for 12 hours until dry, add 100ml acetic acid After dissolving in ethyl ester (at room temperature), add 20ml of distilled water to wash three times, separate ethyl acetate and spin dry to obtain chlorogenic acid with acetylated 4 and 5 phenolic hydroxyl groups.
[0040] The preparation methods of samples 2 and 3 may be similar or close to those in sample 1.
[0041] (2) Tes...
Embodiment 3
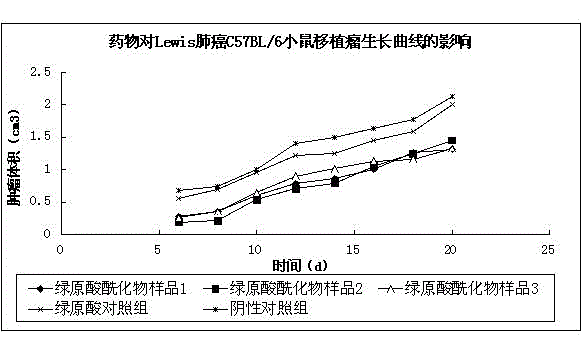
[0063] (1) Animals BALB / C-nu mice, weighing 18-24 g, were provided by the Experimental Animal Center of Sichuan University.
[0064] (2) Drug Chlorogenic acid, commercially available; Chlorogenic acid acylate: R 1 , R 2 and R 3 for H + ions, where R 4 , R 5 for C 2 h 3 o + , marked as sample 1; R 1 , R 2 and R 3 for C 3 h 5 o + , where R 4 , R 5 for H + ion, labeled as sample 2; R 1 , R 2 , R 4 and R 5 for H + ions, where R 3 for C 7 h 5 o + , labeled as sample 3.
[0065] (3) Cell culture SPCA-1 cell is a human lung adenocarcinoma cell line. The cells were routinely resuscitated and passaged. The second-generation mouse tumor was taken, washed 3 times with Hank's solution, blood stains, fat and necrotic tissue were removed, and the tumor was cut into pieces. 1mm×1mm×1mm fragments, rinsed twice with Hank’s solution, added physiological saline (1g:3ml) in proportion, then ground in a glass homogenizer, filtered through a 80-100 mesh screen to make a si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com