Preparation method of ethanol gas sensor component having ultrafast response recovery property
A gas sensor and component technology, applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve problems such as unfavorable low-power device research, inability to achieve real-time monitoring, long response recovery time, etc. Achieve multiple reactive active positions, reduce response recovery time, and respond quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of the ethanol gas sensor element with ultra-fast response recovery characteristics has the following steps:
[0030] (1) Precursor ingredients
[0031] Accurately weigh La(NO 3 ) 3 · 9H 2 O and Fe(NO 3 ) 3 ·6H 2 O;
[0032] (2) Preparation of xerogel
[0033] Dissolve the precursor ingredients in deionized water, add citric acid to form a mixed solution according to the molar ratio of the sum of cations and citric acid at 1:2, and place the mixed solution at 70-80°C (70°C, 72°C, 74°C can be selected , 76°C, 78°C, 80°C) and stir in a water bath, add polyethylene glycol PEG-6000 at a molar ratio of 1:200 to the sum of cations to form a sol, and continue stirring until the dry gel state;
[0034] (3) LaFe x o 3 Preparation of nanopowder
[0035]Put the dry gel into the crucible to heat to remove the organic matter, put the obtained powder into the muffle furnace after grinding, and sinter at 590-610°C (590°C, 600°C, 610°C can be selected)...
Embodiment 1
[0040] (1) Precursor ingredients
[0041] According to the stoichiometric ratio La:Fe=1:0.8, accurately weigh 0.03mol of La(NO 3 ) 3 · 9H 2 O and 0.024mol Fe(NO 3 ) 3 ·6H 2 O;
[0042] (2) Preparation of xerogel
[0043] Dissolve the precursor ingredients in deionized water, add 0.054mol citric acid according to the molar ratio of the sum of cations and citric acid being 1:2, place the mixed solution in a water bath at 80°C, stir and add 2g polyethylene glycol to form a sol, Continue to stir until the dry gel state;
[0044] (3) LaFe 0.8 o 3 Preparation of nanopowder
[0045] Put the dry gel into the crucible to heat to remove the organic matter, put the obtained powder into the muffle furnace after grinding, and sinter at 590-610°C for 2 hours to obtain the LaFe 0.8 o 3 Powder grain size is about 25nm;
[0046] (4) Preparation of side-heated ceramic tube structure gas sensor element
[0047] Take 0.1g of LaFe 0.8 o 3 Put the powder in an agate mortar, add 0.02...
Embodiment 2
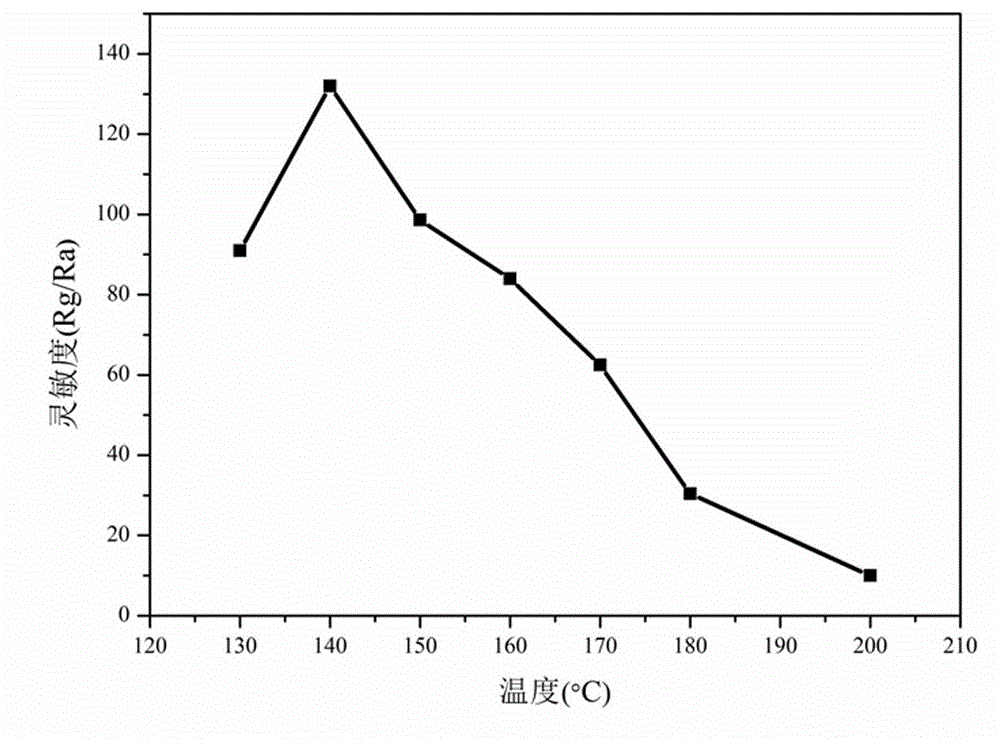
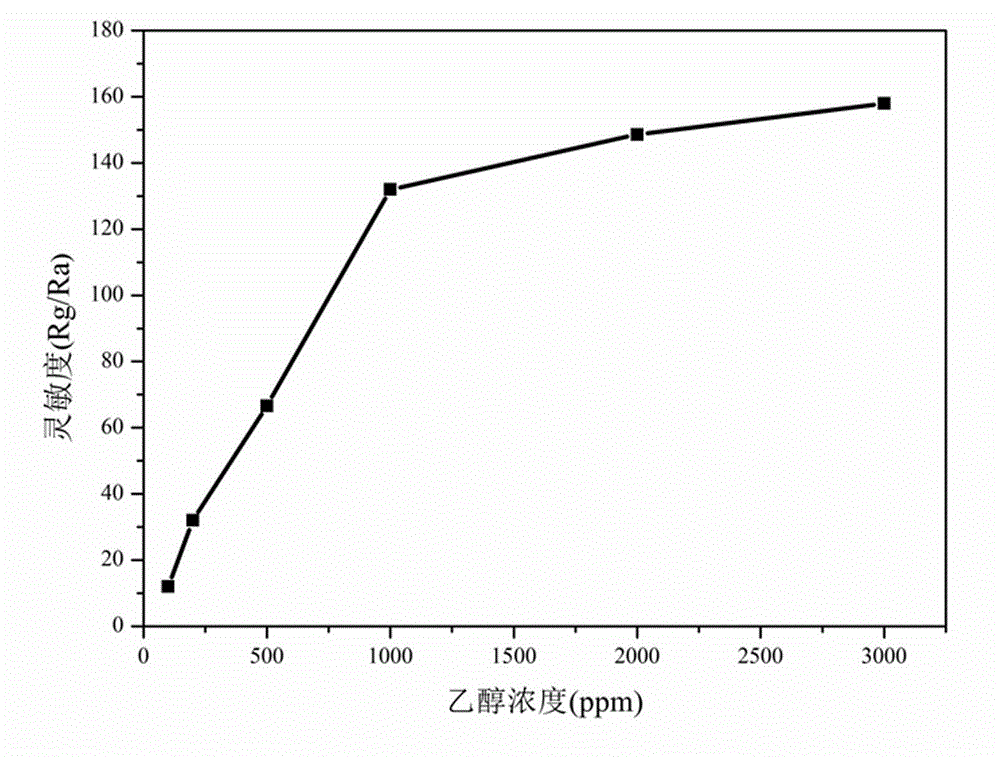
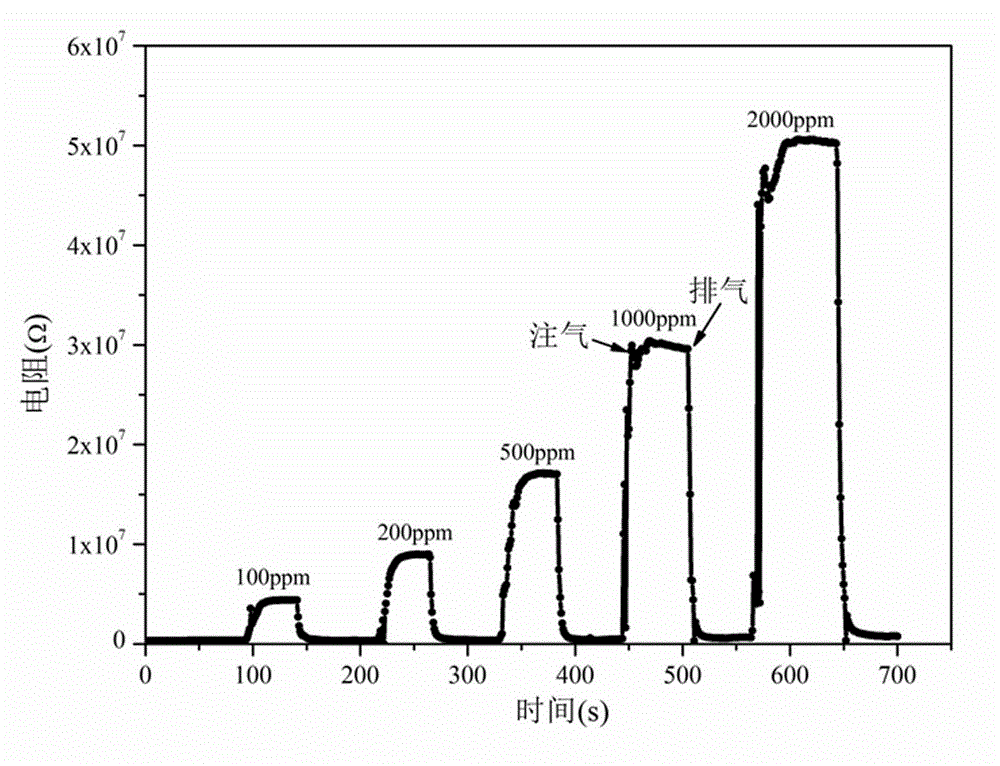
[0050] The difference between this example and Example 1 is that in step (1), the stoichiometric ratio La:Fe=1:0.7 is used for batching, and the obtained nano-scale LaFe 0.7 o 3 The powder grain size is about 20nm, and the LaFe 0.7 o 3 The side-heated ceramic tube gas sensor element has good selectivity to ethanol, the best working temperature and response recovery time remain unchanged, and the sensitivity to 1000ppm ethanol is 95, compared with LaFe 0.8 o 3 Gas sensor elements are somewhat lower, but still better than LaFeO 3 55 high for the gas sensor element.
[0051] The present invention adopts static gas distribution method to measure non-stoichiometric ratio LaFe x o 3 Nanoparticles are the sensitive characteristics of the gas sensor element as the working substance to ethanol gas. The sensitivity of the gas sensor element is defined as S = Rg / Ra, where Rg and Ra represent the resistance value of the element in the gas to be measured and dry air respectively; the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com