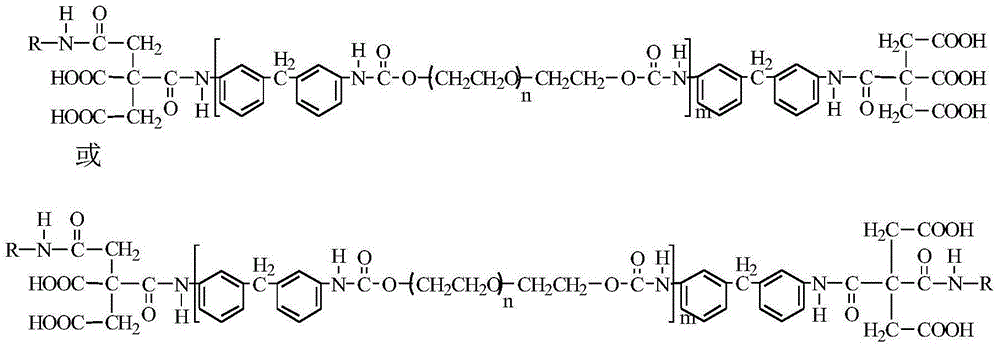
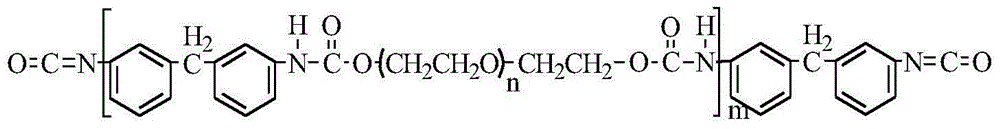Citric acid and chitosan modified biocompatible polyurethane and preparation method thereof
A chitosan modification and biocompatibility technology, applied in the field of biomedical materials, can solve the problems of durability of heparin effect, expensive modification materials, and blood protein pollution on the membrane surface, etc. The effect of improving protein contamination performance, improving anticoagulant performance, and improving chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
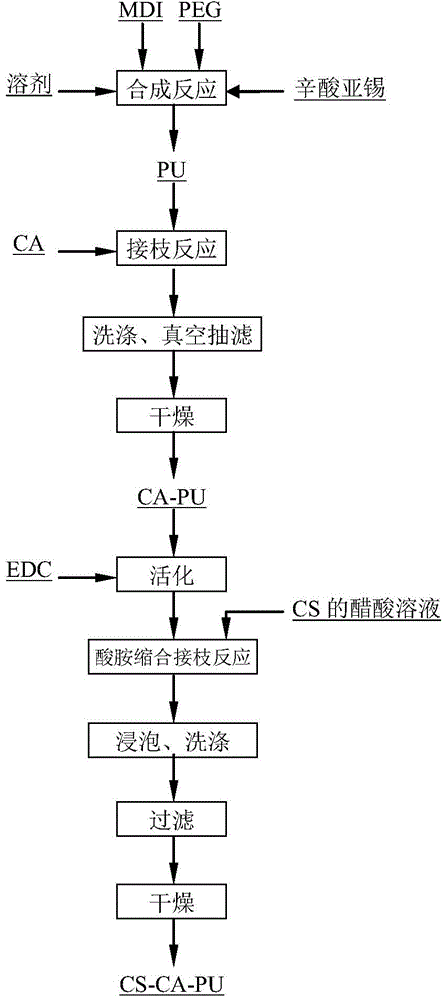
[0034] Add 10 parts (mass, the same below) of diphenylmethane diisocyanate (MDI) and 8 parts of polyethylene glycol (PEG) into a double-necked flask, and then add 42 parts of N,N-dimethylacetamide ( DMAc) solvent and 0.5 parts of stannous octoate catalyst, mechanically stirred until all reactants were dissolved. Under the protection of nitrogen, react at 75°C for 2h to obtain a modified polyurethane precursor (PU). Add 9 parts of citric acid (CA), react at 80°C for 4h; continue to heat up to 85°C for 12h. The product was washed successively with deionized water and absolute ethanol, and dried under constant temperature and vacuum to obtain citric acid-modified polyurethane (CA-PU).
[0035] The above-mentioned citric acid-modified polyurethane (CA-PU) was activated by immersing it in a citric acid buffer solution (pH=4.8) containing 2 mmol / L carbodiimide at 4 °C for 2 h, and then washed with phosphate buffer and deionized water in sequence. , filtered to obtain activated cit...
Embodiment 2
[0037]Add 10 parts (mass, the same below) of diphenylmethane diisocyanate (MDI) and 8 parts of polyethylene glycol (PEG) into a double-necked flask, and then add 60 parts of N,N-dimethylacetamide ( DMAc) solvent and 0.5 parts of stannous octoate catalyst, mechanically stirred until all reactants were dissolved. Under the protection of nitrogen, react at 75°C for 25h to obtain a modified polyurethane precursor (PU). Add 10 parts of citric acid (CA), react at 80°C for 4h; continue to heat up to 85°C for 12h. The product was washed successively with deionized water and absolute ethanol, and dried under constant temperature and vacuum to obtain citric acid-modified polyurethane (CA-PU).
[0038] The above-mentioned citric acid-modified polyurethane (CA-PU) was activated by immersing it in a citric acid buffer solution (pH=4.8) containing 2 mmol / L carbodiimide at 4 °C for 3 h, and then washed with phosphate buffer and deionized water in sequence. , filtered to obtain activated ci...
Embodiment 3
[0040] Add 10 parts (mass, the same below) of diphenylmethane diisocyanate (MDI) and 5 parts of polyethylene glycol (PEG) into a double-necked flask, and then add 42 parts of N,N-dimethylacetamide ( DMAc) solvent and 0.5 parts of stannous octoate catalyst, mechanically stirred until all reactants were dissolved. Under the protection of nitrogen, react at 75°C for 2h to obtain a modified polyurethane precursor (PU). Add 15 parts of citric acid (CA), react at 80°C for 4h; continue to heat up to 85°C for 18h. The product was washed successively with deionized water and absolute ethanol, and dried under constant temperature and vacuum to obtain citric acid-modified polyurethane (CA-PU).
[0041] The above-mentioned citric acid-modified polyurethane (CA-PU) was activated by immersing it in a citric acid buffer solution (pH=4.8) containing 2 mmol / L carbodiimide at 4 °C for 4 h, and then washed with phosphate buffer and deionized water in sequence. , filtered to obtain activated ci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com